Downstream processing continues to present problems for the biopharmaceutical industry in terms of limiting capacity. To address these problems, industry suppliers are actively developing new technologies to improve downstream processing. And bioprocessing facilities continue to seek out and evaluate these technologies. In our 14th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, we assessed the current downstream processing situation by asking 227 industry end-users and 131 suppliers where they see the future trends.
Adoption of new downstream technologies and their ability to head off near-future capacity constraints have a clear impact on biopharmaceutical manufacturing. Growth in the industry has hovered around 12-15 percent annually for well over a decade, and industry capacity has to keep up with that demand. Further improvements in upstream productivity are also creating bottlenecks downstream. But bringing on new technologies can be tricky in this highly regulated industry. Regulating bodies like the FDA and EMA must assess the impact on quality and safety related to production changes, which slows adoption of new technologies, even as the industry need for improvement mounts.
In recent years, upstream processing technologies have made fairly significant advances to help increase capacity and remove bottlenecks in the biomanufacturing system. Partially because of this, the onus is now on expanding downstream processing technologies.
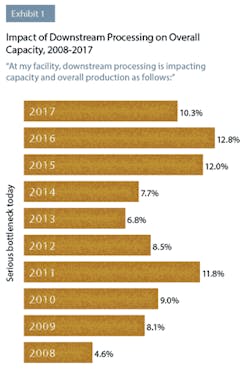
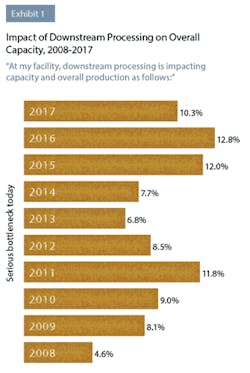
In this year’s study, industry respondents reported that downstream processing was continuing to impact their capacity. This year, 50 percent of respondents to BioPlan’s survey said they were experiencing at least “some bottleneck problems,” compared to 45.7 percent last year. Although not as severe as downstream processing problems have been in the past, clearly this operation area continues to create capacity issues for a large number of biopharmaceutical manufacturers. For example, 10.3 percent indicated this year that they were experiencing “serious bottlenecks today.”
NEW TECHNOLOGIES ON THE WAY
- Membrane technology
- Single use filters
- High capacity resins
- Filters instead of resin chromatography
- Alternatives to chromatography
- Centrifugation
- On-line analytical and control devices
- Countercurrent chromatography
- Precipitation
- 2-phase systems
- Moving beds
- Synthetic biology, enzymatic transformations, etc.
- Field fractionation
- Small substrates
SPECIFIC AREAS OF CONCERN
Other areas of concern included leachates and extractables for single use devices, need for better monitoring and sensors, measuring protein concentration, facility logistics and integrating Process Analytics Technology (PAT).
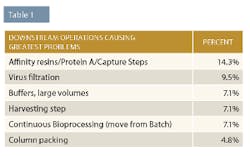
Chromatography problems are typically associated with resins. Industry experts told BioPlan there are too many available, they’re too similar, and they don’t have enough differentiating features. They are hoping to see technological solutions; new affinity formats, ligands, chemistries and resins; new Protein L/mAb fragment resins; more and less expensive custom ligands; and protein A alternatives. End-users want more Protein L and other resins for modified antibody purification, and these may be well-suited for isolation of abbreviated and other smaller engineered versions of monoclonal antibodies.
Column packing creates issues because it is too time-consuming, unpredictable, inconsistent, and costs are too high. Industry experts indicated they would like to be able to use custom pre-packed columns. They also wanted column packing automation and resins that are more packing friendly (rigid).
Lastly, issues arising from clarification/harvesting operations include fouling, complexity/too much variety, and scaling and selection problems. New technologies industry insiders would like to see include flocculation and the ability to painlessly scale up and down their bioprocessing. In addition, development of processes at small and large scales so that the same process is predictable at different scales was also desired.
WHERE THE INDUSTRY HAS MADE IMPROVEMENTS IN DOWNSTREAM PROCESSING
Downstream processing constraints have caused bottlenecks for a number of years. Some continue to be evaluated and implemented. In our study, we asked respondents what actions their facilities have invested in for improvement of downstream processing issues. Top response was: Cycled columns more frequently (39.3% of respondents). Other responses that were above 35% included, “used or evaluated alternative ion exchange technologies,” “investigated single-use disposable downstream technologies,” and “used or evaluated membrane-based filtration technologies.”bio
Interestingly, there are significant differences in which technologies are being implemented between biomanufacturers and CMOs. Over 50 percent of CMOs reported that they investigated single-use disposable downstream technologies (53.8%), while only 33.8% of developers reported the same activity. Likewise, 53.8% of CMOs reported that they used or evaluated membrane-based filtration technologies vs. 32.4% of developers.
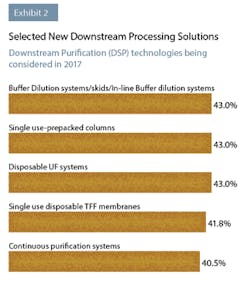
There were also differences in CMOs and developers in what technologies they were considering adopting. CMOs showed the greatest interest in single use prepacked columns (72.7% vs 38.2% of developers), single use disposable TFF membranes (63.6% vs. 38.2% of developers), continuous purification systems (63.6% vs. 36.8% of developers) and single use filters (54.5% vs. 33.8% of developers).
These differences are likely explained by the fact that CMOs are incentivized to adopt new technologies because their needs are more immediate. In addition, they are motivated by cost-savings and the associated need to develop standardized manufacturing platforms. They can also pass related costs on to their clients. And by their nature, CMOs must be able to handle a more diverse and larger number of processes and products. These attributes suggest that CMOs will continue to lead the way in adoption of new downstream technologies to alleviate their bottleneck problems.
BROAD OPPORTUNITIES FOR NEW AND IMPROVED PRODUCTS
In analyzing the annual data, it is clear the bioprocessing community is actively looking for new and better technologies. However, due to the highly regulated nature of the industry, these technologies may require a long implementation period, during which time only incremental changes may be made. Indeed, incremental changes are more the norm than broad sweeping technological revolutions.
The conservative nature of the industry in adopting new technologies is well-founded. Some of the issues include safety/regulations, concerns about capital and operating costs, desires to avoid overly complex technologies, extensive training of staff and changes involve shifting widespread dedication to established technology. Current technologies are, in some cases, decades old. And they work, without causing public health issues. Therefore, a natural avoidance of investing in new technologies has been present in the industry for years.
These issues can be overcome once proof that regulators are on board with new technology is available, and once operating staff are comfortable with new protocols.
SURVEY METHODOLOGY
The 2017 14th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production yields a composite view and trend analysis from 227 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 25 countries. The methodology also included more than 131 direct suppliers of materials, services and equipment to this industry. This year’s study covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world’s major markets in the U.S. and Europe.