Specifying Instruments to Meet Pharmaceutical Hygienic Requirements
Pharmaceutical manufacturing is heavily regulated, though standards designed to guide the selection of measurement and analytical instrumentation for sterile processes are not completely defined. To fill the void, some companies — and even individual plants — have created their own internal guidelines for instrument selection and verification to meet U.S. Food and Drug Administration current good manufacturing practices (cGMPs).
With the need for a comprehensive set of standards clear, the American Society of Mechanical Engineers (ASME) developed bioprocessing equipment standards specific to the requirements of hygienic industries. The resulting ASME BPE (Bioprocessing Equipment) standard addresses materials, design, fabrication, testing and certification.
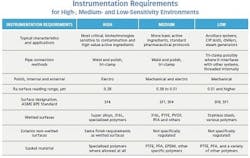
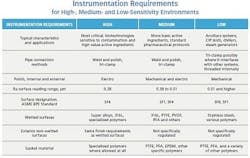
Figure 1: Each environment has specific needs related to equipment configuration and operational practices, which has direct impact on instrumentation selection and configuration.
Prior to the initial ASME BPE release, pharmaceutical manufacturers relied on other sanitary standards such as 3A, first developed for the dairy industry. These were used in conjunction with internally developed guidelines. Unlike the 3A standards, which establish requirements for a relatively narrow set of applications, ASME BPE uses broader applications as a starting point. Over the same time frame in Europe, the European Hygienic Engineering and Design Group developed its own standards to certify equipment and promote effective designs for food and pharmaceutical products.In the past, pharmaceutical manufacturers have been able to pick and choose the criteria required for specific applications, and then communicate the resulting expectations across facilities, suppliers and inspectors. But with ASME BPE, biopharmaceutical manufacturers are finding it easier to coordinate activities and comply with FDA requirements and cGMPs.
ADDRESSING INSTRUMENTATION
The initial ASME BPE release developed general guidelines applicable to instrumentation and other areas, but it wasn’t until 2012 that a specific section was added to cover instrumentation, primarily with respect to bioburden and cleanability. Subsequent updates have added more detailed guidelines for specific instrument types.
Part GR (General Requirements) defines the scope and includes six sections:
1. Manufacturer’s quality programs
2. Inspection
3. Documentation
4. Metrics
5. References
6. Terms and definitions
These general guidelines apply to all types of equipment including process instrumentation,
and also cover some of the less detailed areas. New releases are expected to expand the extent of instrumentation coverage.
Following defined cGMPs, ASME BPE and traditionally accepted practices, users and instrumentation vendors must take into account a number of factors such as:
• Instrument technology type
• Surface design and finish (internal and external)
• Metallic alloy selection (corrosion resistance)
• Non-metallic composition and surface finishes (internal and external)
• Wetted versus external surfaces
• Placement and mounting of instrumentation
• Temperature range requirements
• Suitability for SIP
• Gaskets, seals and welds
• Smart versus traditional instruments
• Need for redundancy
Pharmaceutical plants and instrumentation suppliers have to consider these standards when designing devices for sanitary industries.
TYPES OF PROCESS ENVIRONMENTS
Most pharmaceutical plants have multiple process units to manufacture products. Since there can be many manufacturing approaches used for pharmaceutical products, there are many types of processing units. Approaches can vary from unit to unit with major differences in the measurement, cleanability and bioburden control required for each. There are also varying levels of precision required for processes automation, and concern about potential contamination. In spite of the differences, their degree of importance to the larger operation is often interlinked.
Figure 2a/2b: These are both sanitary instruments, but the one on the left (2a) is designed for the most stringent requirements where even the non-wetted parts of the instrument must conform to hygienic requirements. It fulfills all standards from ASME-BPE, 3-A and EHEDG.
Some processes need to operate in very narrow temperature ranges and within very specific chemical environments to maintain critical fermentation. On the other hand, some basic active ingredients can withstand much wider latitude without affecting critical attributes. Sanitation and the need for cleanliness also vary within the range of basic pharmaceutical requirements, but typically sensitivity to manufacturing parameters and contamination go hand-in-hand.For purposes of this discussion, we will divide processes into high-, medium- and low-sensitivity environments (see Figure 1). Each of these areas has its specific needs related to equipment configuration and operational practices, which has direct impact on instrumentation selection and configuration. These are, of course, generalizations for purposes of comparison and to guide the thought process of selection. Any actual application has to be examined and configured appropriately.
High-sensitivity processes, for example a bio-pharmaceutical fermentation unit, require the tightest control, highest-precision measurements and most sophisticated sanitation and contamination control (Figure 1). These applications call for the highest grade of process-wetted surface finish, BPE SF4. These instruments depend highly on electropolishing rather than mechanical processes as electropolishing provides the smoothest and most cleanable surface with the lowest risk of contamination.
Another way in which this concern for cleanability is manifest is through the regulation of the non-wetted portion of process equipment, as well as the wetted. It is not enough to consider only the side of the equipment actually in contact with the product. Figure 2 illustrates this point clearly. The two instruments illustrated are both sanitary designs and used in pharmaceutical applications. Their wetted elements are identical, but the exteriors of the transmitters are clearly different.
The unit on the left uses the same materials and similar surface finish on the non-process wetted parts as it does on the wetted parts. The expectation is for the entire room to be cleaned. The conventional sanitary unit on the right uses a normal industrial-grade transmitter housing combined with a sanitary sensor.
A medium-sensitivity environment is the level of most conventional pharmaceutical manufacturing. This equipment follows general sanitary practices and is configured for CIP/SIP. The same basic requirements necessary for high-sensitivity areas apply to the product contact surfaces of vessels, piping and instrumentation — but slightly lower surface finish grades are often permissible, and the non-wetted exterior surfaces often do not have to follow the same restrictions. The instrument on the right in Figure 2 is therefore fully acceptable.
However, as a practical matter, any instrument used in this kind of environment will in all likelihood be subjected to wash-down, potentially with a spray-down that can include cleaning chemicals. Instrument housings and wiring need to withstand such treatment on a regular basis.
A low-sensitivity environment exists in most facilities, but these systems are not used for actual product manufacturing, nor do they have any direct product contact. They are the ancillary systems needed to support manufacturing, and are thus not connected to equipment while it is involved in an actual product campaign. Examples could include a tank jacket system.
Figure 3: Using Materials Generally Recognized As Safe (GRAS), as with the inside/wetted surfaces of this Rosemount sanitary magnetic flowmeter, help facilitate instrument leanability and adherence to required sanitary classifications. (All figures courtesy of Emerson.)
Tank jacket utilities are used to heat a reactor or control temperature of the batch and have no product contact. In most facilities, this type of equipment uses normal industrial-grade equipment not subject to the same sanitary restrictions as high and medium sensitivity environments. While this equipment may not have as stringent cleanability requirements as other environments, failure of any of these units can impact actual production processes, so appropriate consideration should be given for reliability and maintainability.INSTRUMENTATION CHARACTERISTICS
The table shows the three different levels of process environments and lists the basic instrument characteristics for each. The same concepts used to guide designs of vessels, valves and piping apply to instruments exposed to the product. Those regulations follow some simple basic concepts:
• Materials must resist corrosion and be easily cleanable — 316L stainless steel with a fine surface finish (Ra surface reading of 0.51 µm or better) is typical. The processes involved in making some active pharmaceutical ingredients are highly corrosive and may demand more exotic alloys, such as those with higher nickel levels.
• Crevices and corners where bacteria and other contaminants can remain must be eliminated — Welds need to be smoothed inside and out, and interior corners must have a radius. Conventional pipe threads are never used.
• Pockets and dead legs in piping are prohibited — Liquids must not have places where they can be trapped or retain residues. Piping should be pitched so it is self-draining, and surfaces should be as smooth and flush as possible.
These basic concepts and others guide instrumentation design. A sensor designed to mount on a pipe tee or tank spud will typically use a tri-clamp for mounting, and the wetted diaphragm has to be as flush as possible (Figure 3). Mounting position matters as well. A sensor may be built following sanitary guidelines and even certified by a relevant standards organization, but if it is mounted incorrectly it may be difficult to clean and may not drain fully.
SMART INSTRUMENTS MAKE SMARTER PROCESSES
As mentioned earlier concerning high-sensitivity environments, construction practices must apply to the wetted parts and even the outside non-process wetted parts of a transmitter. But those requirements are only the beginning. Those kinds of processes tend to be very sensitive and require careful measurement and control. They demand precise and reliable process instruments with a high degree of accuracy and repeatability to ensure reactions happen at exactly the right temperature and pressure. Specific measurements capable of affecting the process are designated as critical process parameters for defined unit operations.
Therefore, smart instruments with sophisticated electronics able to perform self-diagnostic checks are usually applied, especially when monitoring measurements critical to the process. The value of products made in these processes tends to be extraordinarily high, so the risk of losing a batch because of any instrument-related concern including contamination, faulty sensor, or a process incapable of responding quickly to a deviation is enormous.
If alarms programmed into smart devices can warn of a developing process upset before it spoils a production batch, significant financial losses can be avoided. The cost of the most sophisticated instruments, even in redundant arrangements, can be small when compared to ruined product and lost production time.
Many types of process instruments can be specified with these sophisticated capabilities. For example, critical temperature instruments can have dual sensors to create a hot backup mechanism in a single device. The two working together also help ensure reading precision since one measurement can automatically be compared to the other. Smart flowmeters can perform self-analysis to ensure the sensing mechanism is functioning properly. Even something as basic as a pressure instrument can monitor its power supply to verify it can report its full reading range accurately.
Medium-sensitivity environments also require high-precision process instruments, although they might not always call for the same external surface finishes. While products made in these environments may not have the highest values per unit of volume, batches tend to be much larger, and batches lost due to contamination or ineffective process control are still costly. Far worse are the problems related to recalling out-of-spec material once it has been released into the supply chain. Financial costs can be enormous, and a company’s reputation can be harmed.
Pharmaceutical manufacturing applications call for a variety of process instruments to meet the demanding requirements of this highly regulated industry. Users often place their highest emphasis on the configuration of instruments since these are spelled out in detail in the various standards and manufacturing practices. But it is just as important to pay attention to the instrument sensor itself and the instrument transmitter electronics supporting it.