Manufacturing Costs Will Be Critical to Biosimilars’ Success
Biosimilars have finally entered major markets, including the U.S., and are starting to have an impact on the current approximate $200 million biopharma products market. This includes four biosimilars approved in the U.S., while the European Union (EU) has already approved over 20 biosimilars, including some that are capturing big market share from their reference products.
But many companies developing biosimilars ultimately targeted for Western markets are slow with their product development. Some are letting others blaze the regulatory trail with analytical, clinical trials, regulatory approval and marketing. The biosimilars pioneers are also spending substantial resources on biosimilars-related patent disputes. Others are waiting for more approvals and finalization of regulations, especially by FDA, or want more regulatory guidance concerning interchangeability. These biosimilars are very attractive, because they will essentially be A-B generic drug products with full interchangeability and substitution at the prescription/pharmacy level. These products will not require marketing as branded products, as most generic equivalent drugs are sold with no brand marketing at all.
VERY HEALTHY PIPELINE
Despite the delays, many more biosimilars, and new players, will soon be entering markets worldwide as more legacy reference products go off-patent.
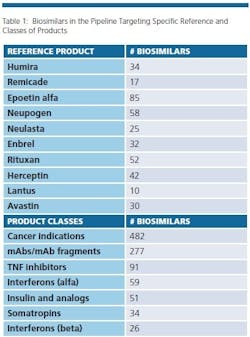
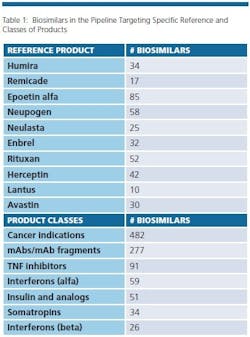
The biosimilars pipeline is healthy. BioPlan’s Biosimilars/Biobetters Pipeline Database (www.biosimilarspipeline.com) currently reports more than 750 biosimilars in various stages of development [1]. The number of biosimilars for major reference products are shown in Table 1. Soon enough, we can expect the number of marketed biosimilars to exceed the innovative biopharmaceuticals. At present, the largest portion of biosimilars are cancer therapeutics, with 482 different biosimilars in the pipeline. More than 100 biosimilars are now in clinical trials. In addition, 277 are either mAbs or fragments. Gaining major market biosimilar approvals is proving to not be too difficult and with negligible development failures so far, a higher percentage of these products in the pipeline can be expected to enter world markets compared to innovative biopharmaceuticals.
Even removing from consideration as ‘biosimilars’ about 200 lower-end (non-GMP) ‘biogenerics’ targeted to lesser- and non-regulated international markets, there are a large number of products in development, with the vast majority targeting the U.S market. This includes biosimilars for nearly every current biopharmaceutical product.
CURRENT LOWEST COSTS FOR BIOSIMILARS APIs
Having low(er) costs for biosimilars manufacturing will be critical. The point of biosimilars is to provide cheaper alternatives to off-patent innovative reference products. Biosimilars must be priced at a discount relative to their reference products, currently ≤30 percent, in Europe; but likely to increase to 50 percent or more, eventually, in the U.S. Biosimilars must also compete with other biosimilars, with 10 or more for each major reference product likely in major markets. Plus they must compete with biobetters and other innovative products targeting the same indications. So there will be a lot of competition, much of it on the basis of prices. Pricing of biosimilars may not always be rational (by conventional biopharma standards). Some developers are expressing intentions to low-ball their prices to capture market share. Others will be interested in protecting or growing their product portfolios and may bundle sales. Competition on prices could well become more extreme as interchangeable biosimilars enter world markets, with these competing directly with the earlier branded biosimilars.
BioPlan Associates recently evaluated costs associated with biosimilars API manufacturing. Bioprocessing professionals with biosimilar developers pre-qualified as knowledgeable concerning bioprocessing costs were interviewed regarding their views on costs currently attainable with biosimilars API manufacturing, particularly monoclonal antibodies. We also evaluated types of manufacturing approaches and facilities most suitable for low costs commercial manufacturing; and what that cost would be for a “typical” mAb biosimilar. As a basis, we presumed that a minimum of 100 kg/year of mAb is required once manufacturing has ramped-up, such as 3-5 years after launch.
Today, biosimilar manufacturing facilities include:
Big (Bio)Pharma 10,000+L bioreactor-anchored facilities
Smaller, flexible single-use facilities
Stainless-steel facilities, mid-scale
Stainless-steel facilities, very large-scale; and
New facilities in developing regions
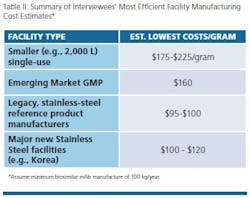
Table II shows general consensus data collected from interviews. Not unexpectedly, the very lowest costs for biosimilar mAb GMP manufacture were reported for facilities operating the very largest scales using stainless steel. This particularly includes Big Pharma legacy facilities with multiple 10,000 L bioreactors. These are followed very closely by the new super-sized facilities coming online — Samsung Biologics and Celltrion, both in S. Korea. The very lowest costs attainable at Big Pharma facilities were reported to be about or slightly below $100 for legacy large-scale facilities. Costs are reported to be just a few percent more for the totally new Korean super-sized facilities.
Despite these low numbers, any facility attaining ≤$500/gram is likely to remain competitive. We note that we have not addressed overall industry average ranges of cost. However, many manufacturers likely will be manufacturing at costs in the $1,000/gram region. As noted, much of the variations in cost for manufacturing are based on scale, but as aspects such as titer improve, the need for massive scale production decreases.
Interviewees noted that even $1,000 or more per gram is still very workable, providing acceptable profits for most biosimilars selling for multiple thousands per dose. For many biosimilar players, it will be of relatively limited impact whether their costs for manufacture are $50 or $500/dose, with their selling prices often in multiple $1000s/dose. Contrary to expectations, best cost/gram for microbial commercial manufacturing were found to be similar to those for mammalian mAbs, while the cost per dose is generally significantly less, with many microbial products substantially more potent than most mAbs.
BEST VALUE VS. LOWEST COSTS
Optimizing biosimilars manufacturing costs is critical for all biosimilar players and, as more products enter the market, costs will increasingly become a determinant in setting price floors. Essentially every interviewee noted that developers are seeking maximal manufacturing cost-effectiveness (within their size/scale and other limits), including adopting current or future-looking, rather than legacy, manufacturing technologies.
Companies that have long manufactured biopharmaceutical reference products were also concerned about their manufacturing costs. With their big vats, and long-established manufacturing platforms, they expect to achieve long-term cost savings.
But most every interviewee noted that having optimized, best fit, manufacturing for products is more critical than simply attaining low costs/gram for APIs. Nearly every interviewee qualified their reporting of lowest costs with biosimilar mAb manufacture by noting that the cost-related variables that really matter most in the long-term involve having a “right-sized” and outfitted facility with optimal scheduling to maximize capacity utilization. If the facility, its processes and scheduling are optimally integrated with cost-effectiveness as the goal, low costs for that type/class of facility will generally follow.
As noted, single-use manufacturing, if done right, can be very competitive, such as attaining costs below $200/gram, along with the inherent flexibility, lower capital expenses, faster turnaround, smaller footprint and other benefits of single-use bioprocessing (but with these operational benefits not usually included in cost calculations). On the other hand, costs in this Single-use range can also be attained using optimally designed, mid-sized stainless-steel facilities, such as the 8,000 L facility Oncobiologics (Cranbury, NJ) designed to support its biosimilar mAbs through initial product launches. It was significant that no interviewees mentioned new stainless-steel facilities as having lowest costs, other than the few new super-sized Korean facilities.
New large-scale facilities in developing countries, notably India and China, were cited as likely having lowest costs averaging around $160/gram. In this context, most interviewees saw no benefits and some downsides involving hidden costs associated with GMP biosimilar manufacture in developing countries. No interviewee presumed manufacture in those countries would be the cheapest. Manufacture in developing countries entails many added costs and difficulties with communications, management, travel, language/translators, shipping delays, unexpected scheduling problems, import duties, safety, costs of foreign consultants, fears of IP/proprietary technology losses, etc., besides the current risks associated with the quality of manufacturing in emerging regions.
Costs of goods/manufacturing, at least as commonly calculated, does not include all of the real costs involved with biopharmaceutical manufacturing. Many interviewees noted that lowest API costs/gram are much less important than total manufacturing operations efficiency, in the context that many aspects critical to bioprocessing and companies are not taken into account in costs calculations. This includes single-use facilities adding considerable value or even being invaluable, such as providing increased flexibility and speed-to-market, factors not included in direct cost calculations. For many, single-use facilities can be preferable and more cost-effective vs. large-scale stainless steel facilities or hiring a CMO. In some cases the increased speed-to-market or lower initial capital investments compensating for somewhat higher calculated costs of manufacturing.
As biosimilars enter major markets, including the U.S, large biopharma companies with the most number of products are the early leaders. However, there are hundreds of companies worldwide developing biosimilars. More biosimilars competition, more products, will coming in most every country and biopharma market niche. The biosimilars pipeline, what is in development, is healthy, with over 750 biosimilars in various stages of development [1]. Soon we can expect the number of marketed biosimilars to exceed the innovative biopharmaceuticals.
Competition will increase, and attaining low costs, generally involving using current vs. older legacy bioprocessing technologies, is critically needed by biosimilar manufacturers to support discounts and defend against the considerable expected biosimilars competition. Lowest costs are generally associated with the very largest scale stainless-steel manufacturing. However, calculated manufacturing costs are just part of the real or total costs of manufacture, which include many hidden costs of simply being a manufacturer.
REFERENCES
Rader, R.A., Biosimilars/Biobetters Pipeline Database.
Rader, R.A., Langer, E.S., “Future Manufacturing Strategies for Biosimilars,” BioProcess Intl.,. 14(5 supplement), May 2016, p. 24-29.