Bridging the Gap Between IT and the Pharma Production Line
In an increasing number of markets, only those companies that meet required standards for serialization and traceability are permitted to sell their products. Pharmaceutical companies can now ensure the future viability of their operations by adding track & trace capability to their business plan.
Gap-free automatic data flow from the corporate ERP system directly to the production line and back is the optimum practice scenario for managers and production supervisors in pharma companies. In this scenario, orders, serial number management and reporting are performed and resolved from the shop floor office to save precious time on the line. Bridging the gap between ERP systems and the line is essential to meeting the international track & trace guidelines of serialization and aggregation. An ERP connection is particularly essential when manufacturers are required to prepare reports with serial numbers used and to register with various institutions.
Mettler Toledo’s XMV is the first step in serialization, applying a code to a package and immediately verifying it with a camera.
A pharmaceutical manufacturer’s production line is controlled by discrete line software. This software communicates with hardware components such as identification and verification systems. The purpose of site management software is to integrate the individual production lines and also connect them to the ERP system. After controlling the hardware components (level 1) and the management of multiple machines on a line (level 2), cross-line level 3 integration is achieved.SITE MANAGEMENT FUNCTIONS
A level 3 software system controls tasks in the network in three areas: order preparation, monitoring and reporting.
1. Order preparation
Thanks to the level 3 connection, production managers can input orders directly into the ERP system and thus plan ahead for several weeks. Instructions for the next order need not be executed on the line itself or in the labeling system, as they can be accomplished from the office or even be automated.
2. Monitoring
Advanced site-management systems monitor all processes related to identification. The PCE Pilot Site Manager, for example, is able to recognize deviations from the norm and can react on its own. A production manager can set specific actions for every possible warning message, after which the system then runs on an automated basis. If the ERP system does not provide enough serial numbers for a new order, then the software will either recognize the mistake and send an email to the responsible employee, or it will automatically request an appropriate number of additional serial numbers.
3. Reporting
The right interface should give pharmaceutical companies full control and transparency of serial numbers. The system should automatically assess the data and create reports about products with serial numbers leaving the production site as well as where the products are being delivered. In certain countries, such as China or Brazil, this information must be communicated to the responsible authorities before the product can be delivered.
If a pharmaceutical manufacturer would like to set serial numbers exclusively in adherence to the 2011/62/EC directive, and is not interested in non-European countries of sale, such as China, then track & trace monitoring can be implemented on the production line with serialization even without the site management system. In this case, a line management system can be used. However, a site management system is essential when there are multiple production lines, a product portfolio containing a broad number of compounds, and different countries into which the products will be sold.
FACTORS INVOLVED IN THE RIGHT SOLUTION
It will be relatively easy for a pharmaceutical company with more than one production line to evaluate the factors that will lead to deciding whether to acquire a site management system — a decision made more critical by today’s continually increasing identification regulations.
Decision-makers from production management and IT together need to consider four factors when weighing the decision and choosing the supplier of the most effective system for their needs:
1. Interfaces
A site-management system, as the connection between the ERP system and the production line, must be compatible with all inventory solutions. The software must have the connectors and interfaces to the ERP solution, the regulatory authorities, the country’s serial number suppliers and relevant stakeholders. In addition, the demands of line management systems and line hardware must match and function together smoothly.
2. Modular architecture
Legal labeling requirements are in upheaval around the world. In many cases, parent aggregation is added to serialization in the range of requirements. Pharma manufacturers must be able to react quickly to changes. And, if the sales department is planning to open new markets, the ability to quickly adjust to new local standards will be required.
Thus, the ideal site-management software is a modular system: manufacturers can add new interfaces, templates and standards without having to reset the entire system. For example, once the core of the PCE Pilot Site Manager is installed, it remains stable; new interfaces and country requirements can be easily added as components. Most important, post-qualification and validation are only required for the new individual components, but are not required for the software core and hardware. This saves a great deal of valuable time and expense when adding these processes.
3. Single source advantage
Experience shows that the more suppliers who are involved in complex system environments, the greater the probability of error. It is therefore extremely important for pharmaceutical operations to work together with a general contractor that can deliver both hardware AND software.
4. Implementation experience
No pharmaceutical manufacturer wants to put itself in the hands of an inexperienced player in such areas as product safety. And they are wise to recognize the danger: delays during roll-outs or insufficient conformity with the latest legal provisions can lead to sensitive supply bottlenecks and expensive compliance violations.
SAFETY WITH FLEXIBILITY
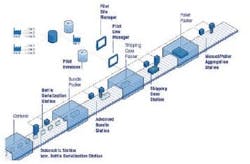
The progression of a package through the Mettler Toledo PCE track & trace system from coding to palletizing.
Pharmaceutical companies sometimes tend to react slowly to new serialization provisions, which results in changes to functioning processes that can increase the complexity of the entire operation. In fact, new provisions provide an excellent opportunity to implement a site-management system to bridge the gap between ERP systems and line components and to increase the overall efficiency of the company. These management systems reduce the effort of serialization and handle the new data complexity behind the scenes. At the same time, they bring the operating benefit of automating many production steps, or handle those steps directly from the office rather than through line operators. In short, a site-management system delivers both the required safety and the desired level of production flexibility.