The biopharmaceutical industry has seen dramatic improvements in upstream manufacturing yields over the past 30 years¹. However, increased efficiencies in upstream operations have contributed to downstream bottlenecks, as these filtration and purification operations have failed to keep pace with upstream developments.
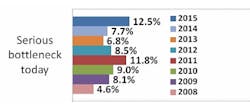
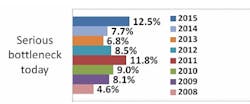
Indeed, according to preliminary results from our industry study, 12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, a majority of biotherapeutic developers continue to suffer capacity constraints as a result of downstream processing. More than seven in 10 respondents (biomanufacturers, excluding CMOs) report experiencing at least minor problems attributed to downstream processing, including more than four in 10 experiencing more serious bottlenecks today.
Source: 12th Annual Report and Survey of Biopharmaceutical Manufacturing, April 2015, www.bioplanassociates.com/11th Preliminary Data
This is certainly not a new problem: Downstream processing has impacted capacity and overall production for upwards of six in 10 biomanufacturers and contract manufacturing organizations (CMOs) surveyed.There's obviously a lot to be gained from improving downstream operations. Separate results from our survey indicate that the impact of improved downstream production operations on biomanufacturing performance at respondents' facilities over the past year has been on par with the impact of respondents' use of disposable/single-use devices. In past years, improved downstream production operations have also been in the top half of factors creating improvements at respondents' facilities.
HOW COMPANIES IMPROVE DOWNSTREAM OPERATIONS
As part of our study, we measure the ways in which organizations tackle the problems associated with downstream processing, asking them which of 19 specific actions they have implemented in the past year to improve downstream purification operations. So far this year, the top five reported activities are:
• Optimizing running conditions (62%)
• Used ion exchange membrane technologies (45%)
• Used membrane-based filtration technologies (44%)
• Cycled columns more frequently (41%)
• Developed downstream processes with fewer steps (41%)
These are similar to biomanufacturers' responses last year, although results to-date this year suggest that companies have been more likely to optimize running conditions.
NEW DOWNSTREAM TECHNOLOGIES IN CONSIDERATION
Aside from tracking the activities implemented by the industry to improve downstream operations, we also measure the new downstream purification (DSP) technologies that respondents are actively considering to address production issues or problems. It's worth noting that this question only asks about active consideration, indicative of potential future adoption, and as a result does not include those respondents already using these technologies or those considering them but not "actively" pursuing them as an interest.
In our results to-date, respondents actively considering at least one of the 22 new DSP technologies we identified, were led by Continuous purification systems (60%); Disposable UF systems (48%); and Single-use filters (48%).
This year's preliminary list of "Most Innovative Downstream Systems" from the 12th Annual Report include:
• Continuous purification systems
• Disposable UF systems
• Single use filters
• Use of high capacity resins
• Single use disposable TFF membranes
• Membrane technology
• In-line Buffer dilution systems
• Buffer Dilution systems/skids
• Single use-prepacked columns
• Alternatives to chromatography
• On-line analytical and control devices
• Use of filters instead of resin chromatography
• Centrifugation
• Moving beds
• Precipitation
• 2-phase systems
• Countercurrent
• Development of MAb Fragments
While these top technologies in active consideration are similar to those seen in last year's study, survey results to-date suggest a greater interest in continuous purification systems, which were actively considered by only 30 percent of biomanufacturers last year. It's also notable that roughly one in five biomanufacturing respondents in this year's study said that they worked with continuous chromatography purification (e.g. simulated moving – SMB) within the prior 12 months to improve downstream purification operations, a figure which would represent a step up from 15 percent in last year's study.
The apparent increase in active consideration of continuous purification technologies this year is interesting, as development of these technologies tends to have lagged behind advances in upstream continuous bioprocessing, with new bioprocessing methods typically pairing continuous upstream processing with conventional batch purification. The lag in adoption of continuous purification potentially relates to its more complex nature, as many more smaller aliquots requires processing. Adoption of continuous purification may also depend on newer chromatography technologies, which are yet to be ready for mass-market adoption.
We're likely to see a focus on this area in coming years, though, as new technologies appear that allow for continuous and semi-continuous operation. The excitement generated by these technologies owes to their potential to enable a jump in the titers processed by downstream operations. Should this come to pass, we could expect to see smaller, more modular and disposable downstream facilities be constructed, ultimately leading to fully disposable facilities, which are currently constrained by the lack of downstream options. Despite their promise, though, we do not foresee continuous purification technologies reaching widespread use at commercial scale this decade.
ALTERNATIVES TO PROTEIN A
An interesting trend that seems to be continuing this year relates to alternatives to Protein A: Although a significant portion of biomanufacturers are investigating alternatives to protein A, few have actively switched to alternatives. Specifically, early data from our study suggests that almost one in five (17%) of biomanufacturers investigated Protein A alternatives during the past year, but just 7 percent switched.
Still, if those figures were to hold true, they would represent a shift from last year's ratio: That year, 33 percent of biomanufacturers had investigated Protein A alternatives, compared to just 59 percent switching. So the percentage evaluating these new chromatography technologies appears to be on the decline.
The high mAb purification efficacy of Protein A resins is one of the attractive qualities of Protein A compared to many proposed alternatives. Protein A resins – now estimated to represent a $400 million per year market - have become increasingly robust and reliable, becoming a dominant downstream mAb purification platform technology. This success and consistent popularity causes difficulties for any improved and/or cheaper alternatives to gain market adoption, although for the time being there remain few cost-effective alternatives. Indeed, while Protein A affinity chromatography has been targeted for replacement from the start due to high acquisition costs and limited recycling, few alternative options have emerged to pose a real challenge to conventional resins.
That's not to say that alternative approaches don't resolve problems (e.g., the high costs, leaching and recyclability). But for them to be successful and gain more rapid adoption, it appears that Protein A resin alternatives will need to display radical rather than incremental improvements.
PUTTING IT ALL TOGETHER
Early results from our 12th annual biopharmaceutical manufacturing survey indicate that biomanufacturers are taking active steps to improve downstream operations, such as optimizing running conditions and evaluating an assortment of technologies. There remain few who have switched to alternatives to Protein A, and recent studies indicate that fewer industry suppliers are working on such alternatives. In fact, the percentage of suppliers last year who indicated that they are working on chromatography alternatives to protein A (12.7 percent) was almost half the share from 2011 (23.4 percent).
Survey Methodology: The 2015 Twelfth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production yields a composite view and trend analysis from over 200 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 30 countries. The methodology also included over 150 direct suppliers of materials, services and equipment to this industry. This year's study covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world's major markets in the U.S. and Europe.
For now, though, technological innovations in downstream purification have yet to lead to the same productivity improvements experienced in upstream operations, as the majority of survey respondents report at least some bottlenecks at their facilities owing to downstream purification issues. It remains to be seen when downstream innovation will make up that deficit.
REFERENCES
1. White Paper: Analysis of Biopharmaceutical Manufacturing: Historical and Future Trends in Titers, Yields and Efficiency in Commercial-Scale Bioprocessing, BioPlan Associates, Inc. July 2014, Rader, RA, Langer, ES
2. 11th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, April 2014, Rockville, MD
www.bioplanassociates.com/11th
ABOUT THE AUTHOR