It is currently a very interesting and dynamic time for the endotoxin testing community, as highlighted at Lonza’s recent Global Endotoxin Testing Summit during which vendors, regulatory bodies, pharmaceutical manufacturers, and conservationists came together to share their thoughts and perspectives on some of the industry’s key issues. Some of the challenges being addressed at the summit and discussed in detail here include methods to overcome the issue of low endotoxin recovery (LER), the need for alternative tests in order to safeguard lysate supply and conserve the horseshoe crab, a guide to validating these alternative assays and the move toward automated systems.
OVERCOMING LOW ENDOTOXIN RECOVERY
Figure 1: The supply of LAL and TAL is reliant on several horseshoe crab species including Limulus polyphemus (shown here) on Pickering Beach, Delaware.
While regulators, pharmaceutical manufacturers and test vendors are aware of the issue, there is still much debate concerning the mechanisms behind LER and how endotoxin masking might be overcome. Allen Burgenson, U.S. Regulatory Affairs manager at Lonza, thinks, “LER is a new type of inhibition. We’ve seen inhibition before and we solved it. There are a number of methods by which LER can be overcome before you have to go into the chemistry of demasking. The issue of LER is manageable, and while I think it is an important technical problem, we do not necessarily have a public health problem.”
Alan Baines, UK Strategic Projects head at Lonza, suggested that, “…when considering new biological license applications, manufacturers should be aware of the potential issues with LER when using polysorbate in combination with citrate or phosphate buffers. Finding the right combination could side-step potential problems. For existing products, it is likely that additional sample treatment steps will be needed to overcome the masking effect, as changes to formulations are usually a much less attractive option.”
To overcome the masking effect, Johannes Reich, PhD student at the University of Regensburg, has been working in conjunction with Hyglos GmbH to gain a better understanding of the aggregation and interaction of lipopolysaccharides in endotoxin testing. His theory assumes that LER is the result of endotoxin aggregate breakdown into monomers, which are subsequently embedded in surfactant micelles. He proposes that this process is reversible and as such, endotoxin demasking will require adjustments in magnesium and calcium, pH and/or the addition of polyanionic dispersants such as Pyrosperse, in order to reassemble the aggregates to allow for sufficient endotoxin recovery.
Figure 2: Lonza’s Global Endotoxin Summit delegates taking part in the Just Flip ‘em program to help save stranded crabs on Pickering Beach, Delaware, one of ERDG’s community horseshoe crab sanctuaries.
However, demasking is still an active area of contention among some industry members as Kevin Williams, senior scientist for Endotoxin Detection at Lonza, believes, “…many people are resisting using the demasking protocol as they want to maintain the simplicity of the test. I can certainly understand. However, you can’t maintain simplicity by denying that complexity exists. So what we hope to do is to inform and educate at least the manufacturers of biologics such as monoclonal antibodies about the importance of addressing LER, as these are life-saving drugs and it’s a significant and growing market area.”This reluctance is understandable, as carrying out an investigation into LER could delay the release of new products and cause significant financial issues if a product cannot be released within a reasonable timeframe. This is a particular problem for biological products, as they often have a very short shelf-life and require a fast turnaround time.
Regulators can also add additional time pressures; if a product is tested and shown to display LER, manufacturers will need to overcome the issue within a given timeframe, as stipulated by the FDA (typically one year but this can vary). The manufacturing delays introduced by the need to overcome the issue will likely be significant. In addition, biological products for which no defined hold-time has been established will need to be tested as soon as possible. This may cause a back-up in QC testing, as products that exhibit the signs of early or somewhat instantaneous LER will need to be pushed ahead of other products where a longer hold-time has been established. If no clear hold-time can be established during the development stage due to LER, significant time and money may need to be invested to uncover better formulations that exclude the excipients known to be associated with LER.
The threat of LER can also influence manufacturing efficiency and cost in other ways. For example, in some cases, manufacturers may feel the need to implement alternative testing strategies, such as the introduction of additional in-process analysis steps during the manufacturing stage, or even at different points in the process where LER is not yet a factor. Such changes to existing workflows can have significant operational and financial impacts. Lastly, the FDA requires extensive evaluation of the drug product regardless of whether LER is present, which could be time-consuming and costly.
Evidently, further investigation in this area will ensure a better understanding of the LER and the means by which this issue can be overcome in a timely and cost-effective manner.
THREATS TO NATURAL AMEBOCYTE LYSATE
The Limulus or Tachypleus Amebocyte Lysate (L/T-AL) assays are currently the most widely used bacterial endotoxin testing (BET) methods. The need for these tests is on the rise due to an increase in the demand for medicinal products, including biopharmaceuticals, particularly as more countries become economically developed. All parenterally administered pharmaceuticals will inevitably need to be tested for bacterial endotoxins and as such arise in vaccine production could place strain on lysate resources if current habits remain unaltered.
The supply of LAL and TAL is reliant on several horseshoe crab species namely, Limulus polyphemus (Figure 1) and Tachypleus gigas or Tachypleus tridentatus, respectively. This is because when endotoxins come into contact with the blood of these crabs, a clotting cascade is activated. It is this mechanism that has been used for endotoxin testing since its commercialization in the 1970s.
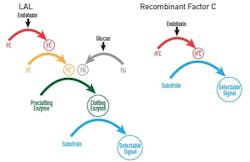
Figure 3: Endotoxin-activated clotting cascade comparing LAL assays and Pyrogene recombinant Factor C Assay (Lonza).
As the need for the amebocyte lysate increases, so too does the need to protect the crab populations. In North America, this has meant that the Atlantic States Marine Fisheries Commission has implemented a state-by-state fishing quota and the conservation efforts of the Ecological Research and Development Group (ERDG) such as Just Flip ’em and Backyard Stewardship community horseshoe crab sanctuary (Figure 2) have been put in place to safeguard the native L. polyphemus populations. However, in Asia a lack of regulation has meant a considerable reduction in indigenous T.gigas and T. tridentatus numbers. A collaborative effort from regulatory bodies, pharmaceutical industry, vendors, end-users and conservationists will aid in ensuring horseshoe crab populations are preserved. In addition, the development and adoption of alternative testing methods that do not rely on a potentially finite animal resource could also help to achieve this.ALTERNATIVE ENDOTOXIN TESTING METHODS
Alternative methods, such as Lonza’s PyroGene recombinant Factor C (rFC) assay, use a synthetic form of Factor C, the essential component of the endotoxin-activated clotting cascade (Figure 3). Not only do such alternatives negate the need for an animal resource, but also they offer several other benefits over the natural assays. These may include ease-of-use, lot-to-lot consistency, statistically robust spike recovery and enhanced endotoxin specificity.
Despite these benefits, many are reluctant to use these alternatives. This is due to the fact that both the FDA USP <1225> and EP Chapter 5.1.10 stipulate additional validation steps to be conducted when using these methods. However, pharmacopeial members, like Ingo Spreitzerare keen to discuss how rFC-based tests are defined. “I hope to see increased usage of rFC in the field of endotoxin testing. This shouldn’t be too challenging in my opinion, as I don’t see a significant difference between the LAL assay prepared from crab and industry prepared rFC — both depend on Factor C.”
Furthermore, the validation procedure can be accomplished in 1-3 days and doesn’t require much additional effort compared to compendial methods. The process is simplified by the availability of documentation and well-structured protocols from test manufacturers that can help users generate sufficient data to meet the regulatory requirements. Allen Burgenson thinks, “We’ll need big players to adopt rFC so that others will follow and this will increase the chance of it being accepted by regulatory bodies,” if alternatives are to become common practice.
MOVING TOWARD AUTOMATED TESTING
Automating the endotoxin testing process could help improve efficiency and manage the increase in demand. Alan Baines believes, “Automation of the preparation steps will be possible. In fact, for large-scale users this has already been successful, but this is expensive for the application and out-of-scale with the need of the assay. Testing is moving from the QC laboratory to the manufacturing floor, with the hope that this will allow problems to be detected sooner and prevented at an earlier stage. This has had some limited success so far, but this could become much more prevalent over the next 10 years.”
Wolfgang Mutter, general manager of Hyglos GmBH, concurred saying, “If we have the technology I think we can manage this.” However, if the regulators do not account for the technological advances, this could mean that “…rather than using the latest biochemistry, such as rFC, new automation processes will be designed using LAL reagents, even though they could soon become obsolete.” For this reason, it is imperative that the pharmacopeias be kept up-to-date on developments in endotoxin testing in order to ensure the guidelines for use are acknowledged in the compendia. As a result, this will increase the probability of these methods being taken into consideration when designing new instrumentation.
MODIFYING PHARMACOPEIAL GUIDELINES
New products challenge the current pharmacopeial guidelines. Ingo Spreitzer envisages, “New types of products are going to affect the future of endotoxin testing. I predict that we will need to develop a much more detailed and product-specific set of risk assessments in the future.” Therefore, in order to ensure the industry and regulators move forward in parallel, every stakeholder will need to be involved to ensure a rapid mutual progression amongst the endotoxin testing community.
LOOKING TO THE FUTURE
The endotoxin testing community is increasingly aware of the imminent transformative period ahead. Hence, regulatory bodies, pharmaceutical manufacturers, reagent vendors and horseshoe crab conservationists will need to work together to align goals and set clear objectives to ensure innovative methods, which improve upon current assays, are incorporated into the pharmacopeia and adopted in the QC laboratory. Change is inevitable throughout this process. Therefore, it is necessary to have a responsive system in place to ensure prompt implementation of alternative tests while still maintaining patient safety, which remains the utmost priority for the pharmaceutical industry.
Given the research currently underway to fully understand LER and to assess potential solutions, it is likely that the problem will soon be solved and without becoming a risk to human health. Endotoxin testing may be improved upon if the industry can be encouraged to carry out the additional validation steps required by the regulators when using an alternative method, as these tests deliver comparable results yet offer a number of additional benefits for the user in addition to reducing the strain on lysate supply and protecting the horseshoe crab populations.
Evidently, change is coming and the global endotoxin industry will need to adopt a collaborative approach to realize sustainable BET operations and ensure patient health and well-being remains at the forefront of all future endeavors.
ABOUT THE AUTHOR
Lakiya Wimbish, product manager for Endotoxin Detection at Lonza is responsible for alternative methods like the PyroGene™ recombinant Factor C Assay, Testing Services and Rapid Microbial Detection platforms. She joined Lonza eight years ago as an Applications Development Scientist for Rapid Microbial Detection platforms before transferring into her current marketing role. Prior to Lonza, she was a contractor for the United States Navy/Biological Defense Research Directorate and graduated from the University of Pennsylvania.