Pharmaceutical manufacturers and similar life sciences organizations must keep current, complete records of all submissions and communications associated with drug applications submitted to regulatory agencies — both directly and through their affiliates. Access to complete records of exactly what was submitted wherever a company has applied to conduct business is essential. This includes related communications such as emails, meeting minutes and phone records. These documents are typically scattered across a variety of electronic document management systems (EDMS), laptops, collaboration spaces, email and shared drives — making a comprehensive view challenging.
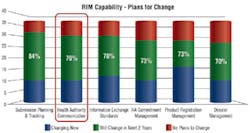
Figure 1. In a recent survey, 78 percent of respondents said they were looking to change the regulatory information management (RIM) system they use for health authority communication.
The tools typically used for the archiving and retrieval of these items simply cannot meet today’s business requirements. For example, companies need to respond quickly to agency queries, requiring immediate access to relevant, up-to-date information. In addition, regulatory compliance dictates that records are protected and access controlled — functionality not normally available with methods such as fileshare sites.But there’s a better way; namely implementing a purpose-built solution. For example, when working through this process, what if all of your regulatory submissions for medicinal products over time (paper, non-eCTD and eCTD) were centralized and easily searchable, allowing you to view all regulatory documentation and related activity at one time? What if you could look at various submissions for a product, select the one of interest and see all the submissions, queries, telephone calls, meetings and email exchanges along a single timeline?This article will compare the two main ways of managing submissions to regulatory authorities: using fileshare tools and email, or with a purpose-built solution. Although the first method is perhaps more widely used, it has its distinct limitations. But before we look at methods for managing the records for regulatory submissions, let’s examine the industry’s current state of affairs in this area.INDUSTRY RECOGNIZES CHALLENGES
The submissions management process is sure to increase in complexity. To get ahead of the curve — and in an effort to improve overall efficiency — companies are beginning to look for alternative approaches to managing regulatory information and communications.
In fact, the recent report “Managing Regulatory Information as a Corporate Asset — Industry, Health Authority and Vendor Trends” makes it clear that life sciences companies are revising their business and technology plans for global submission management. The report, based on an industry study conducted by Gens and Associates Inc., highlights an increasing focus over the next several years on health authority communications. As managing partner Steve Gens explains, “… global expansion is requiring consistency of interactions across regions. … The ability to provide consistent information across global health authorities is critical and difficult to achieve.” Survey responses showed that only 40 percent have an authoritative source for health authority correspondence, indicating a need for additional IT investment in this area.
Figure 2. Regulatory submissions for life sciences plants must be prepared, submitted and tracked on a regular basis.
Thus, the drive toward a much more dynamic approach to information and communications management is an understandable development. “Global submission management is predicted to be the top area of change over the next two years,” according to the report. Figure 1 from the survey shows the areas forecast for greatest change.To meet today’s complex business requirements, you will need a 360-degree view of all submissions, along with fast access to agency correspondence concerning every product that your company has in the market. Agency representatives will continue to assume that you, the sponsor of a given product, have visibility into everything submitted for that product, along with complete access to previous queries. And because most organizations store regulatory correspondence across multiple repositories, you will need a comprehensive view of all agency interactions in chronological order (Figure 2).
To fulfill these requirements, many companies have defaulted to email and fileshare tools, which are readily available, but more often than not have considerable limitations.
EMAIL AND FILESHARE ISSUES
Today, the most common way to share business information within a company, and among a company and its partners, is via email and fileshare platforms. With this method, information is shared among parties through email transmittals and fileshare sites. Emails are typically used for sharing smaller scale attachments such as Word and Excel files, while fileshare sites are used for larger attachments as these often exceed email system limits.
The main advantages of sharing information using email and fileshare sites are their familiarity, ubiquity and low cost. Virtually everyone is used to working with these basic tools, and most every company has the necessary software installed for email communications. Fileshare sites range from free to very low cost, although costs can climb rapidly as required features are added.
But sharing data via these methods has some serious inherent limitations, particularly for life sciences companies with respect to regulatory submissions. Fileshare sites provide a convenient, consolidated location for pushing a submission to an agency through a gateway or via physical distribution. All current commercial publishing tools send their output there as a default. But these sites are intended to be temporary locations, not an archive of the submission. This approach prevents compliance with key business requirements, for instance, securely controlling all submission information for easy and rapid recall.
And there are other serious limitations. For example, these repositories are not capable of protecting the integrity of a submission with role-specific, version-control functions and audit trails. This functionality is necessary to comply with 21 CFR Part 11 for electronic records.
With email and fileshare tools, numerous agency queries and response documents, meeting materials and telephone conversation records are saved in emails, shared drives, laptops or collaboration tools. Responding to inquiries and keeping track of communications between the sponsor and the agency can quickly spiral into a complex, unorganized mess — with hours wasted searching multiple systems, and with repeated calls to affiliates asking for the latest information or status. Integrating all related correspondence thus becomes a serious challenge.
When you combine the constraints of email and fileshare tools along with highly fragmented correspondence management, the result is duplication of effort, inefficient business processes, and lack of confidence in the quality of information
PURPOSE-BUILT SOLUTION
There is a way to overcome the limitations of email and fileshare tools, but it does require IT investment for software purchases, host hardware and training. This method relies on software running on the sponsor’s servers, generally the life sciences company. This software can be internally developed, but it’s more common to purchase a software solution from a third-party.
• Works with information from a variety of sources
• Simplifies storing and viewing of submissions
• Speeds access to submitted information and related correspondence
• Highly secure
• Access controlled
• Built-in regulatory compliance features
To realize the advantages listed in the table, the selected purpose-built solution should have the following capabilities:
• An integrated chronological view with correspondence linked to the submissions, providing an at-a-glance look at all interactions to date.
• Import and store current as well as legacy submissions in eCTD (electronic common technical document), NeeS (Non-eCTD electronic Submissions) or paper formats.
• Make submissions navigable and viewable from the repository with functionality equal to a regulatory review (enabling the sponsor to see the submission exactly as the reviewer sees it).
• Provide standard eCTD views — single, current and cumulative — of the full regulatory submission lifecycle, including navigation of hyperlinks.
• Out-of-the-box integration with Microsoft Outlook, allowing users to drag and drop emails and attachments directly into the repository for automatic import.
• Archive submissions for long-term retention while providing easy information search and rapid recall, fully in compliance with 21 CFR Part 11 for electronic records.
• Makes possible true closed-loop reporting between affiliates and sponsors so everyone knows exactly what was submitted, to whom and when.
• Search for archived submissions via faceted navigation or based on metadata properties such as product, country, submission type, manufacturer or date.
The correct solution should have all these capabilities, and it must also be flexible, dynamic and rapidly responsive to changing business conditions and requirements. For example, if regulatory agency requirements change, the solution should offer software updates to facilitate compliance.
STRINGENT NOW AND LATER
Regulatory submission requirements are stringent today, and will become increasingly so in the future. There are two main ways to meet submission challenges, but the default email and fileshare approach has serious limitations. Pharmaceutical and life sciences companies are therefore looking to implement purpose-built solutions, as these have the capability to meet regulatory submission requirements, now and in the future. These solutions are available in the marketplace and have existing successful implementations, so a good path forward is to contact and evaluate relevant supplier solutions.
ABOUT THE AUTHOR
Steve Scribner is a consultant with over 40 years of experience in computer solutions implementation in business. For the past 20 years, he has focused on electronic document/content management systems in the Pharmaceutical / Life Sciences industries.
REFERENCES:
1. “Managing Regulatory Information as a Corporate Asset; Industry, Health Authority, and Vendor Trends;” Gens and Associates RIM Whitepaper: http://gens-associates.com/wordpress/wp-content/uploads/2014/10/Executive_RIM_Whitepaper_Gens_and_Associates_Fall_2013_Edition_release.pdf
2. Steve Gens, Gens and Associates Inc., and Greg Brolund, Chicopee Falls Consulting, LLC, “Managing Regulatory Information as a Corporate Asset—Industry, Health Authority, and Vendor Trends,” 2013.