Pharmaceutical manufacturers continue to operate in an increasingly competitive environment, contending with issues that run the gamut from lost revenue from expired, patents, ballooning costs for new drug research and development, changes required by new regulations and other compliance mandates and global market pressures to reduce costs and improve quality and delivery.
The quality control (QC) laboratory plays a critical role in pharmaceutical production, for both in-process and finished product testing. Labs not only monitor and control the quality of incoming APIs (active pharmaceutical ingredients), and other supplies used in the manufacturing process; QC Labs are also instrumental in the batch release process.
As a result, steady pressure is on to improve QC lab operations. In general, the challenge boils down to this: finding a way to improve capacity and utilization of resources, reduce lead times while increasing reliability, and speed up the authorizations required for compliance; for both production and batch release.
Lean thinking provides useful ways to address the challenge. However, while lean has been used extensively in many discrete and process manufacturing industries, laboratories have lagged behind in applying lean principles. But they are starting to catch up. Many of these same principles can work in virtually any laboratory environment, including medical and clinical laboratories, as well as laboratories in other types of chemical manufacturing.
THE QC LABORATORY ENVIRONMENT
QC laboratories play a crucial role in the manufacturing value stream for pharmaceutical products. But lab environments have their own special characteristics. In fact, labs share many aspects of both manufacturing and service operations, in a unique combination.
Labs are like super-clean manufacturing environments, where significant attention must be paid to both safety and compliance. Equipment is often highly sophisticated, extremely sensitive, and very expensive; proper operations and maintenance are essential to avoiding equipment breakdowns. Labs are internal suppliers to the pharmaceutical manufacturing process, but like many service operations, their processes and “products” – timely test results – are mostly intangible and invisible in comparison with those of manufactured goods. Processes often take place in a “black box.”
Maximizing both staff time and machine time is essential in the lab, as is standardizing work to ensure that protocols are followed. In reality, though, it’s all too familiar to find laboratory technicians and managers continually grappling with:
• Variable demand and uneven workloads
• Complex scheduling that combines routine testing with special tests and projects
• Individuals who are their own mini-silos; that is, they are set up to work to their own schedule or “drumbeat” rather than to the rhythm of demand
• Large backlogs and missed deadlines, which trigger fast-tracking or expediting of work and further complicate scheduling
• Plenty of conformance, but not much real control
What’s lacking is two-part:
• The top-down, managed work of standardizing processes, of analyzing the nature of demand and how it matches with resources, and of finding a better way to effectively manage flow and scheduling; and
• The bottom-up, self-managing processes of visual management and lab organization, skills development, and teamwork.
THE IMPETUS = THE NEED FOR SPEED
To illustrate how a specific strategic change can serve as the springboard for a lean transformation, let’s consider the case of one international pharmaceutical company we’ll call Fine Pharma. The impetus for launching a “lean lab” at Fine Pharma began with a change from in vivo to in vitro testing on a key (and very expensive) product line. In addition to the social and ethical benefits associated with reduced animal testing, in vitro testing would also be much faster, and much less expensive after the amortization of new testing equipment.
According to Catherine Converset, president of Productivity Europe and senior consultant with Productivity Inc., the change brought a big challenge: “Everyone was used to working with the long lead times associated with in vivo testing,” Converset said. “The impact on the whole lab was significant, as everyone had to get used to the shorter lead time and learn to work differently.” Establishing a lean laboratory environment would help reduce lead time and stabilize the QC process so that the company could effectively use a quicker batch release process.
LEAN LAB TRANSFORMATION OVERVIEW
There are two broad dimensions in a lean lab transformation. The first is improvement of the whole value stream for different product families, from receipt of incoming materials to shipment of finished products. The QC laboratory needs to be viewed as part of the larger manufacturing value stream—by both production and lab personnel. It’s not uncommon for QC labs to be seen as separate departments, with no real understanding of how lab operations affect production or vice versa. Making changes in this dimension starts with seeing and analyzing those relationships, typically using value stream mapping to understand the macro processes.
The second dimension is transformation of operations inside the laboratory to:
• Establish a lean scheduling system for both people and machine time, based on a clear understanding of volume and demand variation;
• Reduce changeover time between assays;
• Improve workplace organization and create standards;
• Establish visual flow management;
• Develop effective performance management systems, and
• Create a team and problem-solving culture.
A key element of this second dimension is the process of establishing a “visual laboratory.” That’s a lab in which staff can see and visually manage standards for operations, demand and the flow of processes, the organization of materials and supplies, and performance measures.
THE VISUAL LABORATORY
Creating a visual lab involves three key steps:
1. Creating stability in the workplace and in processes;
2. Linking processes in flow; and
3. Managing the workload visually.
Taken together, they help create the team and problem-solving culture that makes a lean lab transformation effective and sustainable.
CREATING STABILITY
Workplace stability begins with using 5S to organize the laboratory. 5S is a systematic way of creating a workplace that can be visually managed, using the five seemingly simple principles of “sort, set in order, shine, standardize, and sustain.” When used effectively 5S principles have an enormous impact on operations, including safety.
Applying 5S starts with staff acknowledging that the lab is a shared environment for which everyone is responsible; that’s often a key cultural shift that needs to be reinforced through daily behaviors. 5S techniques are used to organize specific items in specific locations in specific quantities, so that any item can be located in 30 seconds or less. Everyone is charged with adhering to 5S and process standards, and the lab environment is managed visually, saving time and frustration. Safety is improved through consistent labeling, handling, storage, and management of all kinds of lab equipment and supplies, including solvents and other active and reactive products that can be hazardous.
Process stability is achieved through standardization. That goes beyond traditional compliance thinking about standardizing the “what’s” to be done, and extends to the “how’s.” Standardizing allows lab staff to manage their processes visually by creating a visual process standard for how each assay is carried out, regardless of who is performing it.
People stability comes as a result of cross-training and multi-skilling. In labs, staff are used to associating specific, qualified people with the skilled tests that they specialize in performing. In a lean lab, processes must be considered separately from the people needed to perform them, and the company works toward the ideal of training everyone to perform every assay according to process standards so that the lab can respond flexibly to demand.
“Since strict standards must be met, you frequently find that individuals become specialized in running certain types of analysis,” Converset said. “The problem is that if you really want to be flexible, you need multi-skilled people. Very often in labs that is not the case, and that’s one of the challenges.” It’s not necessarily an easy challenge to meet, but it can be done, and the rewards in flexibility are significant. At Fine Pharma, lab technicians were successfully cross-trained in how to perform both chemical and microbiological testing and interpret the results. “In the end, it was not that different,” Converset said, “but people get used to doing one type of analysis. If you do not do a certain type of analysis regularly, you forget how to do it. So you have to keep rotating tasks in order to keep up the practice of responding to demand flexibly.”
LINKING PROCESSES IN FLOW
Visual labs focus on the flow of samples through the laboratory rather than on individual assays, technicians, or pieces of equipment. To support that flow focus, lean labs define a shared and visible scheduling system that provides reliable lead times and incorporates both routine and non-routine tests as well as scheduled time for administrative work, meetings, and special projects.
The work of QC labs can be extremely complex, especially in labs that handle a variety of different types of testing. Creating an appropriate scheduling system on a case-by-case basis is a must to optimize the time of both people and machines in different environments. Labs can have short machine cycle times and long manual times, the opposite, or a combination. The process of creating a simplified scheduling system can be quite challenging.
A lab schedule that handles complexity visually. This board was developed from a kaizen event to review process delays. Availability of the right person at the right time was a problem. The team split the day into 15-minute increments and used color-coding to denote types of activities. The value stream of the process (green activities) takes priority; other activities have to be scheduled around it; and the staff can easily keep track visual of what needs to be done.
“In every lab,” Converset said, “you have to discover a new logic to shorten lead time. In manufacturing processes, you usually have one scheduling system for a production unit. But in a lab you might have three or four different sub-labs, and you have to build the best-adapted system for each. In addition, it’s not just that people need to be multi-skilled in all the labs if you want flexibility—they also need to be able to transition into the logic by which you do the scheduling for each. That can be complex.”
Creating a schedule based on the pull of demand begins with understanding the true nature and volatility of that demand, usually by analyzing it over a given time period, and then creating routines that help level out the peaks and valleys. Combined with a multi-skilled staff and a visual method for tracking progress, labs can see big improvements in throughput times and on-time releases.
MANAGING THE WORKLOAD VISUALLY
A visual planning board can be used to allocate incoming assays in a standardized way to a leveled lab schedule. That provides a way to manage the workload visually. At a glance, staff can see lab capacity, can see the work in process and work to be done, and can see whether they are working to demand. People become used to working to the demands of the process and their internal customers, rather than in silos.
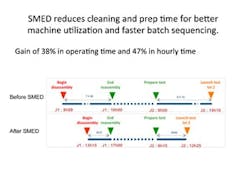
Improvements in changeover times between assays, before and after SMED (single-minute exchange of die, or quick changeover) techniques were applied.
Through standardized visual scheduling, one lab was able to increase the number of HPLC (high performance liquid chromatography) assays performed in a 10-day period from two to three. Because two technicians are required in the process—one to perform the assay and one to verify—the schedule was revamped to assign two technicians rather than one to the core process. Each technician now worked half-time on HPLC assays and half-time on other duties. The result was a 50 percent improvement in HPLC productivity with no additional staff hours.
OTHER KEYS TO THE LEAN LAB
Reducing changeover times between assays is a critical part of improving capacity. The principles of reducing changeover time in manufacturing operations can be applied directly to lab operations to improve turnaround times. Using 5S principles and establishing changeover kits can help enormously. And the gains can be significant. At one QC lab at Fine Pharma the team saw reductions of 38 percent in operating time and 47 percent in hourly time after applying quick changeover techniques.
A visual performance board makes the tracking of key indicators visual.
In addition to managing the workload visually, it’s important to establish a way to manage performance visually, in order to monitor the key parameters of QCD: quality—conformance to regulatory requirements; delivery—conformance to customer requirements; and cost—conformance to budgetary requirements. Departmental performance boards that display this data in trend charts over time are instrumental in establishing visual indicators that everyone can see and relate to.
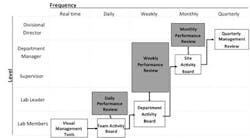
Visual trend charts must be used in the context of a visual performance review system that is regularly adhered to. An ideal visual performance review system cascades upward, starting with the visual management tools used in real time by lab team members, up through the monthly and quarterly performance review information used by departmental and division managers.
RESULTS
Following the implementation of visual lab and other lean techniques, QC laboratories have seen excellent results including:
A visual performance review system that cascades upward, from lab team members to top managers.
• Lead time reduced from more than 6 weeks to less than a week.
• On-time delivery improved from 75-80 percent to 98 percent or more.
• Reductions in changeovers, resulting in improvements of 40-50 percent in operating time.
• Productivity improvements of 50 percent or more.
• Paperwork reductions of 80 percent.
• Significant improvements in staff morale and teamwork.
Applying lean effectively in the QC laboratory environment requires a good understanding of how the lab functions in the context of the pharmaceutical manufacturing value stream, of the work culture in a specific laboratory and company environment, and of how lean is applied in both manufacturing and service environments. When used effectively, lean principles can yield enormous productivity improvements in QC labs, improvements that are sorely needed in the current pharmaceutical environment.