Implementing a Continued Process Verification (CPV) program requires overcoming obstacles manufacturing companies typically face when they evolve their traditional systems. Leveraging technology solutions can help break through such barriers to ultimately make it easier to access and use process data for trending, automated alerts and improved process understanding and business benefits.
For the past few years since the FDA updated its guidance for process validation, many life sciences manufacturers have been adopting new programs. Process validation is defined as the collection and evaluation of data, from the process design stage throughout production, which establishes scientific evidence that a process is capable of consistently delivering quality products.
Process validation involves a series of activities taking place over the lifecycle of the product and process. The FDA describes the process validation activities in three stages: process design, process qualification and CPV, which is the “ongoing assurance (that) is gained during routine production that the process remains in a state of control.” The FDA emphasized in its document that process validation should not be thought of as a singular event, but as a program requiring ongoing demonstration that a process remains in control.¹
The products and processes of Baxter International Inc.’s BioScience business are varied, but they share the common connection of improving the lives of patients with rare conditions, chronic diseases or limited treatment options. Commissioning its Global Information Technology team to lead the charge, Baxter knew it could capitalize on the following business benefits through an automated CPV program:
• Improved Data Access – Access to data is generally achieved through a few subject matter experts (SMEs) who know where and what the data is and how it relates to a process. CPV programs formalize this knowledge so that more people have self-service access to the data with a common “data dictionary” used for reference and to guide access to the data.
• Better Control – Data is frequently extracted into multiple spreadsheets or data and sources. A CPV program can reduce the number of data sources and formalize control processes by creating a single point of self-service access to data that provides a single version of the truth.
• More Integrated Data – Data must be integrated with its context (e.g., data type, genealogy, lot number, date, batch number, process step, etc.) to be understood as information that drives action. Most frequently this context is provided personally from the knowledge of SMEs. CPV can formalize and automate this data contextualization so that scarce and expensive SME time can be allocated to more value-adding activities. Additionally, there is a desire to relate the information from different sites and systems, and CPV can optimize this type of integration through one point of access.
• Automation of Routine Process and Product Monitoring – In-process and final product parameters can be monitored automatically rather than manually. This provides a high assurance of product safety with evidence from every batch that all parameters are operated within their desired manufacturing limits, not just the final product specifications.
• Automated Alerts for Review-by-exception – Monitoring by exception through an automated CPV program provides operating staff and support teams with automated out-of-trend (OOT) alerts, so they can take action before batches begin to fail. This allows technical experts to spend their time only on those process parameters that are trending outside of pre-set limits rather than reviewing hundreds of trend charts to catch just those few that require immediate attention.
• Validated Environment – Baxter’s CPV program included systems that conformed to the FDA’s 21 CFR Part 11 requirements and Good Automated Manufacturing Practices (GAMP) to ensure its electronic record data capture, as well as the rest of the software systems, are validated and, therefore, trustworthy and reliable for decision-making without the need for the additional expense and risks of second person verification tasks.
• Data More Easily Re-used – Data is frequently extracted for a single use, and each time the data is needed by different people it is extracted again and calculations/derivations are made repeatedly. Through CPV programs, data and key calculations/derivations can be stored for more efficient re-use.
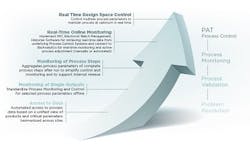
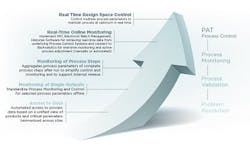
The CPV program roadmap to real time monitoring and control.
Baxter wanted to implement a global management approach across multiple sites, to improve process understanding and control process variability across its entire network of manufacturing assets. With its new automated CPV program in place, the company expected to identify yield improvement opportunities through process comparison within a site and/or across different manufacturing sites. It wanted to better understand the correlation between process parameter and quality indicating outputs, especially the Critical Process Parameters (CPP) and Critical Quality Attributes (CQA). This would allow trending of parameters, so teams could take action before a specific factor became a problem, thereby avoiding costly deviations and product discards.
Ultimately, Baxter could switch from a data collection and reconciliation mode to an analyzing and decision mode of operation. For the future, Baxter knew its CPV program could enable real time process monitoring and control as Figure 1 illustrates.
OVERCOMING BARRIERS
Baxter’s CPV program is known internally as BioAnalytics, and it consists of integrating Operations Intelligence (OI) and guided input from Manufacturing Operations and Quality departments. The vision is to establish efficient manufacturing processes and enable the production of high quality products for patients. The company set out to create an automated analytical capability based on a unified view of products and critical parameters harmonized across its global manufacturing network sites to improve process knowledge and control.
Baxter defines OI as the process of bringing together operations data from many sources to generate process knowledge that will drive improved results. OI elements include automated transfer of data from the shop floor, aggregation of data from multiple sources, providing context and structure to the data and providing capabilities for standard and ad hoc analysis and reporting. All of these elements were considered essential for a collaborative CQV program.
Like other life sciences companies, the nature of Baxter’s business presented inherent barriers and special considerations for implementing a CPV program, including:
• Accessing process and quality data stored in both electronic and paper systems
• Improving data analysis/understanding process variability
• Making comparisons across global manufacturing network
• Changing the culture/adapting participants to new habits.
When Baxter began its CPV implementation program and related technology enablers, the team used an incremental approach for each product and manufacturing site to overcome short-term challenges while working toward long-term goals. It defined how technology enablers mapped to each of the three process validation lifecycle stages.
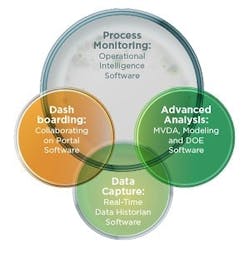
The BioAnalytics program team is responsible for implementing the global harmonization of the tools shown in Figure 2. Baxter wanted to implement a global software system that could provide access to all manufacturing data, including, but not limited to, process control systems, in-line device recordings, results of laboratory quality assays, manufacturing execution data and other batch record data. It needed analysis and reporting capabilities including the creation of control charts, product and stability trending, and other statistical analysis based upon selected data and automation or routine updates and alerts. And, last but not least, it wanted the ability to compare results across all plants and at the overall divisional level.
Baxter BioAnalytics tools portfolio is harmonized throughout its global BioScience manufacturing network.
The team borrowed change management methodology from author Brien Palmer who said, “All improvements to how we operate as a business require change, and all change causes a predictable resistance by those people who are affected by the change. Unfortunately, this tendency — the lack of acceptance of the change — often causes a project to fail, even if the desired change is perfectly logical and necessary.”²
To help overcome this “human” barrier, the team outlined organizational requirements, assigning each department’s role in the CPV transition and program across Quality Operations, Manufacturing Operations, Global Information Technology and Technical Services and Process experts. Individual sites were prepared for the BioAnalytics implementation by assessing the current readiness and defining actions to sustain and utilize the accompanying systems and processes.
The implementation of BioAnalytics relied on the availability of validated underlying systems that contain the data, such as online data/historians, ERP software, process control systems, electronic batch management (EBM) batch release workflow and in-process and final product quality record management software.
Among its lessons learned, Baxter’s team recommends identifying process champions to drive the project, and ensuring early on that the team is well defined and utilizes change management tools and assessments to make necessary corrections and support the implementation. Gauging realistic time commitments from SMEs helps focus resources on the project without distractions from competing priorities. SMEs who can make a difference — and who are often the key beneficiaries of the use of the product — can rally the team and follow up with consistent messages to ensure the overall vision and common goals are always present.
Integrating Baxter’s global and local teams was an important step. Local site resources were increased to ensure continuity and accommodate related, increased demands. They increased local flexibility by having a local Six Sigma Black Belt on site and an IT representative, who was empowered to support local change management activities, develop changes and adjust configurations, for example. At the global level, the team established peer reviews for configurations and directed changes through an operational steering committee on a quarterly basis.
To date, Baxter’s BioScience business has mapped thousands of parameters for product development, process monitoring or investigational purposes. It has implemented its BioAnalytics program for three of its products. Eight sites have started implementations, with three using the program for product manufacturing.
Time savings across projects and routine production have been mapped to easily available process monitoring data. Opportunities to reveal previously unknown processing elements and new CPPs, along with early trend detection that helps avoid out-of-spec (OOS) results have positively affected yields. These benefits, together with a better availability of data, time spent on investigations has been reduced by 80%, encourages Baxter to move forward along its path toward real time monitoring and control.
References
(1) FDA Guidance for Industry, Process Validation: General Principles and Practices, January 2011, CGMP, Revision 1.
(2) Palmer, Brien, “Making Change Work: Practical Tools for Overcoming Human Resistance to Change,” ASQ Quality Press, 2003.