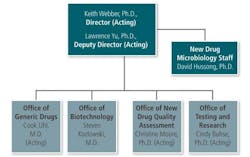
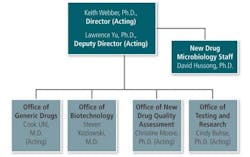
In a recent webcast, (acting) Deputy Director (OPS) Lawrence Yu stated that the concepts embodied in the QbR (Question based Review) approach, now used in ANDAs will be extended to NDAs in 2014. There are several reasons for this expansion:
1. There is a large backlog of applications, some of which is due to each company using its own format for a NDA. Using a “template,” even only with high-level questions, will speed the process by enabling reviewers to find and evaluate the sections they need to find.
2. Many companies acquire their APIs from 3rd party manufacturers and no longer make their own. Both the ideas in ICH Q11 and QbR extend the responsibility for API characterization to the company that makes the final product. Blind acceptance of CoA is no longer an option. This puts “branded” drugs in the same vulnerable position as generics with regard to potential problems with API purity.
What Dr. Yu was saying is that the additional requirements will become official in 2014. He was explaining how the super-department will streamline the review and inspection approaches for “branded” and generic companies. This will, if nothing else, allow NDAs and ANDAs to be evaluated more judiciously and allow the “good” products to market sooner than later.
The new OPQ, working with ORA, will work as a combination of an industrial Quality Assurance department and an “overseer” for the new departments under OPS. There have been complaints that generics are treated differently than proprietary drug companies (strangely, each claims the other is favored in inspections).
Maybe I am just being naïve, but I find this reorganization exciting, especially since Drs. Yu and Woodcock are insisting that QbD is alive and well and being emphasized under the new organization. They may come up with a new (and hopefully improved) acronym, but I will always refer to the process as QbD. Watch this space for more developments.
Published in the October 2013 edition of Pharmaceutical Manufacturing magazine