Auditing of suppliers is a universal practice among biomanufacturers. As the industry matures, however, we are seeing an increase in the amount and types of auditing being done, as a result of the industry’s increased focus on productivity and performance. In the 8th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production [1], we found that almost half of biomanufacturers are auditing more of their suppliers and secondary suppliers (with nearly all presumably already auditing key vendors).
We studied the quality management techniques being used by global biomanufacturers to assure quality of supply. Today’s evolving biopharma environment requires that biologics developers pay increasing attention to quality management issues to avoid production problems, capacity bottlenecks, and failures. An integral facet of this oversight is the supply chain: End-users are seeing more importance in supply chain management, and are increasingly attempting to take control through managing, assessing and protecting their supply of materials.
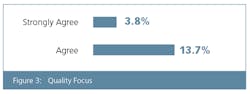
Our annual survey, with responses from 352 global biomanufacturers, asked respondents to identify what, in the past 12 months, their organization had done to assure consistent quality in raw materials and ingredient supply. Not far behind “audited more of our suppliers,” which was cited by 49% of respondents, was “audited our suppliers more frequently,” at 45% of respondents.
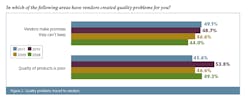
These numbers are not entirely unexpected—supply chain management and oversight are becoming more essential, particularly as the industry continues to adopt single-use/disposable bioprocessing equipment. Here, bioprocessing equipment is repeatedly purchased, used and disposed of, rather than being permanently installed and recycled. These repeated purchases place more stress and importance on the supply chain. Vendors and equipment suppliers (and, in turn, their materials, parts and component suppliers), must be in full compliance with regulatory and related documentation requirements. (See Figure 1.)
References
1. 8th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production: A Survey of Biotherapeutic Developers and Contract Manufacturing Organizations, BioPlan Associates, April 2011, 490 pages.
About the Author
Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD in 1989. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing”, and many other industry reports. [email protected] 301-921-5979.
Survey Methodology: The 2011 eighth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production in the series of annual evaluations by BioPlan Associates, Inc. yields a composite view and trend analysis from 352 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 31 countries. The methodology also encompassed an additional 186 direct suppliers of materials, services and equipment to this industry. This year's survey covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world's major markets in the U.S. and Europe.