Air-Coupled Acoustics: Sound PAT for Tablet Characterization
Physical (mechanical) properties and mechanical defects (e.g., cracks, capping and delamination) of drug tablets may affect their therapeutic functions1,2. Here, a non-contact/non-destructive acoustic technique for determining the mechanical properties such as Young’s moduli, Poisson’s ratios and mass densities of the core and the coat materials and coating thickness of tablets with mono-layer coating layer is described. These properties are critical to the design of tablets and the performance evaluation of the relevant manufacturing processes. The current method is based on air-coupled excitation of a tablet and the interferometric detection of its vibrational motion.
Many modern commercial tablets consist of two basic structural parts: the core and coating layer(s). Since the core of a typical tablet contains a mixture of one or more active pharmaceutical ingredients (APIs) with a number of inactive excipients, containing diluents, binders and lubricants, we consider a tablet as a mechanical drug delivery device consisting of bonded functional and structural parts (e.g., core and coating layers). For instance, it has been known that the Young’s modulus of a material compacted into solid dosage can often be related to its mechanical hardness3, and hard tablets may impact disintegration time4,5 and thus the release rate of the medicament in the digestive tract, potentially affecting therapeutic response. For many drug products administered in solid dosage form, tablet coating thickness is an important critical-to-quality (CTQ) attribute of the final product6. Tablets are coated to control release of active ingredients in the body, to avoid irritation of the esophagus and stomach, to protect the drug from oxidation and hydrolysis (from humidity in the air), to provide a barrier to unpleasant taste or odor and to protect the stomach from high concentrations of active ingredients. Also, coatings can provide an aesthetic or identification function, provide mechanical strength to the tablet core and in some types, such as enteric coats, improve drug effectiveness and stability and regulate and/or extend dosing interval. Mechanical properties of the core and the coat materials and coating layers play a key role in drug bioavailability, stability and shelf life of a tablet. Cracked or damaged coating could subject the patient to hyper-therapeutic levels of drug. With the advent of new tablet types such as osmotic pumps, push-and-pull and multi-layered tablets, the mechanical structures of some solid dosage forms have become quite complex, requiring complicated tablet architecture as well as patient-friendly administration. Therefore, these types of tablets require more exacting testing and monitoring techniques and faster information return rates than possible with existing techniques.
Quality Control and Quality Assurance (QC and QA) functions play a major role in every industry; however, their effects in the pharmaceutical industry are very prominent since drug formulations are used to treat patients. There are stringent regulatory requirements that must be met in order to market the drug. Also, the regulatory approval process has become significantly more complex and costly. The regulatory agencies are more sophisticated, capable, cautious and conservative in evaluating drugs than in the past. Consequently, their standards for approvals are significantly higher. This, in turn, is forcing pharmaceutical companies to test their new treatments in larger, more comprehensive and more costly clinical trials6. As a result, the demand for measuring and evaluating the mechanical properties of tablets and for monitoring tablet quality has been increasing. For example, for comprehensive quality assurance monitoring in the pharmaceutical industry, the U.S. Food and Drug Administration (FDA) has initiated a guidance program, Process Analytical Technology (PAT)7, which is defined as a system for designing, analyzing and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final pharmaceutical product quality. Such procedures would be consistent with the basic tenet of “Quality by Design” and could reduce risks of quality and regulatory concerns while improving efficiency7. PAT encourages8 the use of acoustic techniques2,9,10 as well as other critical techniques including NIR11, acoustic emission AE12, light-induced fluorescence LIF13, Terahertz-pulsed spectroscopy14, laser induced breakdown spectroscopy (LIBS)15, x-ray fluorescence method16 and Fourier transform infrared (FTIR) spectroscopy17 for characterization and monitoring of tablets by using on-, in- and/or at-line measurements.
Compared to the characterization and monitoring techniques mentioned above, the non-contact/non-destructive acoustic technique detailed here could have potential advantages in testing and evaluating the mechanical integrity of the core and the coat materials and the coating layer thickness of tablets such as suitability for rapid (microsecond-scale time scale) on-line characterization and coating thickness determination applications that eliminate the time lag associated with off-line techniques18,19 and can be cost-effective compared to other measurement techniques13-17.
The Experiment
To demonstrate the utility and effectiveness of the presented non-contact characterization technique, a set of 15 tablets (commercially available) with the average mass of 477 mg and with the characteristic dimensions of 6.32 mm thickness, 11.35 mm diameter and a coating thickness of 135 μm are employed in the experiments (Fig. 1).
Figure 1. The dimensions of the tablet with its top (a), side views (b) and the microscope image of the cross-section of the tablet (c) at 100×. The tablet thickness is determined as 135 µm.
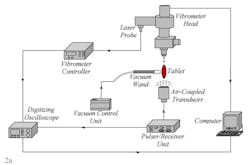
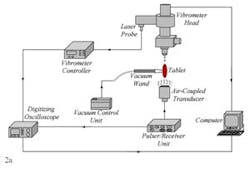
Only the results of four tablets with measured coating thicknesses of 135.1 µm, 136.1 µm, 135.8 µm, 136.2 µm are presented here. The proposed non-contact/non-destructive experimental set-up incorporates a square pulser/receiver, an air-coupled transducer, a laser interferometer, a vibrometer controller and a digitizing oscilloscope, as well as a vacuum handling apparatus consisting of a vacuum wand and a vacuum pump with a suction power of ~30 kPa. The instrumentation diagram of the acoustic excitation and interferometric detection set-up and the tablet mounting apparatus for acquiring transient responses of tablets and the image of the bottom excitation configuration with a vacuum wand holding the tablet in place are depicted in Fig. 2a and b.
The first step of the characterization procedure is to determine a set of vibrational resonance frequencies of the tablet. The pulser/receiver unit excites an air-coupled transducer with a square pulse at a central frequency of 120 kHz (Fig. 2a). The tablet is sonified by an acoustic field generated by the air-coupled transducer. The propagating acoustic field excites a number of vibrational modes (harmonics) of the tablet. The tablet surface transient responses at a number of points on the tablet surface are acquired by the laser interferometer in a non-contact manner by detecting the shift of a reflected laser beam. Acquired transient responses are processed to determine the resonance frequencies of the tablet.
Figure 2. The instrumentation diagram of the acoustic excitation and interferometric detection set-up and the tablet mounting apparatus for acquiring transient responses of drug tablets (a). Image of the bottom excitation configuration with a vacuum wand holding the tablet in place (b). Surface vibrational responses are captured by the laser interferometer in a non-contact manner by detecting shift of a reflected laser beam.
Material Properties from Resonance Frequencies
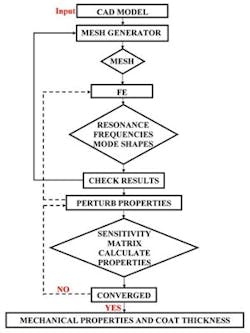
The second step is to extract the mechanical properties of the tablet core and the coat and the coating thickness from the measured resonance frequencies of the tablet. Resonance frequencies and corresponding mode shapes of a tablet are related to its mechanical properties (e.g., Young’s moduli, Poisson’s ratios and material mass densities of the core and the coating layer) as well as its geometry (e.g., shape and dimensions of the core and the coating layer). Using an FE algorithm, resonance frequencies can be obtained provided that the mechanical properties and geometry of the tablet are available.
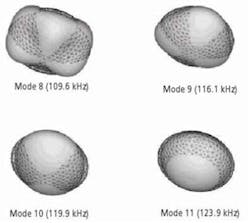
In the FE study employed to compute resonance frequencies of the tablet, a three-dimensional mesh for the tablet is modeled as homogenous and isotropic elastic solid consisting of a core and a coating layer for numerical predictions of the tablet resonance frequencies. The number of elements, number of nodes and degrees of freedom are 9,013, 16,965 and 19,987, respectively. The characteristic element size of the coated tablet mesh is 100 µm, which is comparable to many of the pharmaceutical powder particle sizes (e.g., microcrystalline cellulose MCC (Vivapur 102) particle size 90 µm – 100 µm). Modeled tablet resonance frequencies are obtained in the frequency range of 40 kHz to 200 kHz (Figure 3), which is consistent with the bandwidth of the air-coupled transducer used in the experiments (100 kHz – 150 kHz). The mechanical properties (Young’s modulus E, Poisson’s ratio v and mass density ρ) of the core and the coat of the tablet and coating thickness are then extracted using an iterative computational procedure such as Newton’s method as well as from its resonance frequencies, as detailed in2.
Figure 3. The four vibrational mode shapes of the tablet.
Results
After extracting the mechanical properties and coating thickness for each tablet Pexc, the FE method is employed to determine the corresponding resonance frequencies for extracted mechanical properties and coating thicknesses ƒFE to compare with ƒexp. The reported data indicates that, due to tablet-to-tablet variations, 0.2%, 0.2% and 1.1% differences are detected in mechanical properties, coating thicknesses and resonance frequencies, respectively, among the four tablets. The percent error between the experimental resonance frequencies ƒexp and the FE resonance frequencies ƒFE corresponding to extracted mechanical properties and coating thickness Pexc is within ±0.8% for the four tablets.
This proposed air-coupled acoustic technique requires no physical contact with the tablet and operates in the microsecond time-scale; therefore, it is suitable for rapidly determining and evaluating the mechanical properties and coating layer thickness of tablets. Also, the ability of the acoustic waves to penetrate the tablet surface and to vibrate entire tablet structures expands the abilities of the proposed non-destructive/non-contact testing platform for micron-scale defect detection in tablets, millimeter-scale layer thickness determination in bilayered and multilayered tablets, active ingredient drop content measurements and micron-scale multiple layer coating thickness determination in tablets.
Figure 4. Flow chart of the iterative process for extracting the mechanical properties of the coat and the core of the tablet from the measured tablet resonance frequencies.
References
1 Cetinkaya, C., Akseli, I., Mani, G.N., Libordi, C.F., Varghese, I., et al., Non-Contact Mechanical Characterization and Testing of Drug Tablets. In: Advanced Ultrasonic Methods for Material and Structure Inspection. ISTE Science and Technical Publishing, London 316-369 (2006).
2 Akseli, I., Cetinkaya, C. Drug Tablet Thickness Estimations Using Air-Coupled Acoustics, International Journal of Pharmaceutics. 351: 165-173 (2008).
3 Fell, J.T., Newton, J.M. Determination of Tablet Strength by Diametral-Compression Test. Journal of Pharmaceutical Sciences. 59: 688-691 (1970).
4 Indiran, P.S., Russell, I., Syce, J.A., Neau S., et al., Sustained Release Theophylline Tablets by Direct Compression Part 1: Formulation and In Vitro Testing. International Journal of Pharmaceutics. 164: 1-10 (1998).
5 Saravanan, M., Nataraj, K.S., Ganesh, K.S. The Effect of Tablet Formulation and Hardness on in Vitro Release of Cephalexin from Eudragit L100 Based Extended Release Tablets. Biological and Pharmaceutical Bulletin. 25: 541-545 (2002).
6 Steiner, M., Bugen, D.H., Kazanchy, B., Knox, W.T., et al. The Continuing Evolution of the Pharmaceutical Industry: Career Challenges and Opportunities. Regent Atlantic Capital (December 2007).
7 Hussain, A.S., et al., Foreword, Journal of Process Analytical Technology. 1: 3 (2004).
8 PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. http://www.fda.gov/cder/guidance/6419fnl.pdf.
9 Varghese, I., Cetinkaya, C. Non-Contact Photo-Acoustic Defect Detection in Drug Tablets. Journal of Pharmaceutical Sciences. 96: 2125-2133 (2007).
10 Li, C., Cetinkaya, C. Frequency Domain Thickness Measurement Approach for Microscale Multilayered Structures. IEEE Transactions on Instrumentation and Measurement. 55: 206-211 (2006).
11 Kirsch, J.D., Drennen, J.K. Nondestructive Tablet Hardness Testing by Near-Infrared Spectroscopy: A New and Robust Spectral Best-Fit Algorithm. Journal of Pharmaceutical and Biomedical Analysis. 19: 351-362 (1999).
12 Serris, E., Perier-Camby, L., Thomas, G., Desfontaines, M., Fantozzi, G., et al. Acoustic Emission of Pharmaceutical Powders during Compaction. Powder Technology. 128: 296-299 (2002).
13 Lai, C.K., Zahari, A., Miller, B., Katstra, W.E., et al. Nondestructive and On-line Monitoring of Tablets Using Light-Induced Fluorescence Technology. AAPS PharmSciTech. 5: 1-10 (2004).
14 Fitzgerald, A.J., Cole, B.E., Taday, P.F. Nondestructive Analysis of Tablet Coating Thicknesses Using Terahertz Pulsed Imaging. Journal of Pharmaceutical Sciences. 94: 177-183 (2005).
15 Mowery, M.D., Sing, R., Kirsch, J., Razaghi, A., et al. Rapid At-Line Analysis of Coating Thickness and Uniformity on Tablets Using Laser Induced Breakdown Spectroscopy. Journal of Pharmaceutical and Biomedical Analysis. 28: 935–943 (2002).
16 Behncke, H.H. Coating Thickness Measurement by the X-Ray Fluorescence Method. Metal Finishing. 82: 33-39 (1984).
17 Ryan, J.A., Compton, S.V. Brooks, M.A., Compton, D.A. Rapid Verification of Identity and Content of Drug Formulations Using Mid-Infrared Spectroscopy. Journal of Pharmaceutical Biomedical Analysis. 9: 303-310 (1991).
18 Podczeck, F., Drake, K.R., Newton, J.M., Hariran, I. The Strength of Bilayered Tablet. European Journal of Pharmaceutical Sciences. 29: 361-366 (2006).
19 Stanley, P. Mechanical Strength Testing of Compacted Powders. International Journal of Pharmaceutics. 227: 27-38 (2001).