Demystifying GMPs for Nutritional Supplements
By Cynthia Kradjel, Integrated Technical Solutions, Inc.
This June (2008), the estimated $25 billion US dietary supplement industry passed a major milestone: the first FDA deadline requiring companies with more than 500 employees to be compliant with dietary supplement cGMPs. Mid-sized companies, with less than 500 employees, have until June 2009, while small companies of less than 20 employees have until June 2010 to implement the requirements of this regulation, which is notably distinct from pharmaceutical GMPs and food GMPs.
The dietary supplement industry appeared on the radar screen when the Dietary Supplement Health and Education Act (DSHEA) passed into law in 1994. DSHEA amends the Federal Food, Drug, and Cosmetic Act of 1938 (FDCA) to alter the way dietary supplements are regulated and labeled.
DSHEA defines “dietary supplement” by law in Section 3 of the Act to mean “a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: (A) a vitamin; (B) a mineral; (C) an herb or other botanical; (D) an amino acid; (E) a dietary supplement used by man to supplement the diet by increasing the total dietary intake, such as enzymes or tissues from organs or glands; or (F) a concentrate, metabolite, constituent, extract or combination of any ingredient described in clause (A), (B), (C), (D), or (E).”
According to DSHEA, a dietary supplement is a product that is labeled as a dietary supplement and is not represented for use as a conventional food or as the sole item of a meal or the diet. The definition describes the variety of forms — capsule, powder, softgel, gelcap, tablet, liquid, or other form — by which these products can be ingested.
In addition to laying the foundation for a regulatory framework for dietary supplements and their ingredients, DSHEA, under Section 9, provides FDA with the authority to promulgate good manufacturing practice (GMP) regulations for supplements. In 2003, almost ten years after DSHEA was passed, the guidelines for the dietary supplement GMPs were published for review and comment. Four years of discussion and controversy led to the final regulation being published in June 2007. The outcome establishes standards that require that dietary supplements are manufactured consistently as to their identity, purity, strength, and composition. It seeks to ensure that they are not adulterated with contaminants or impurities and are labeled accurately to reflect the active ingredients and other ingredients in the product.
Some History
Prior to the dietary supplement GMPs, many suppliers had rigorous programs to self-police their manufacturing, but others did not, simply relying on the certificate of analysis (COA) to verify quality of the incoming raw materials. The recent example in the pet food industry of melamine adulteration in wheat gluten highlighted just how dangerous it is to solely rely on the COA parameters for overall quality assessment. Melamine was added to give false positive results for protein value in the Total Kjeldhal Nitrogen method.
Without guidelines, a wide range of manufacturing standards were applied, leading to extremes in overall quality. Consumers purchased products that had positive impact, others appeared to have no effect and a small number had negative impact. Third party testing labs publicized findings that, while some products were true to label claim, some had less active than label claim and in extreme cases some products contained no active at all. Unethical producers cut corners on processed ingredients that were not what they were purported to be. Articles appeared in the media (such as “Get What you Pay For”, Food Processing, September 2000) about false positive HPLC testing results of chondroitin sulfate and FTNIR spectral pattern recognition being used to flag the adulterated material. Manufacturers who built quality into their processes had to justify higher prices compared to manufacturers who cut corners. The industry itself worked to self-regulate for its own survival. Stories of herbal products on the market being discovered that actually contained undisclosed synthetic adulterants or prescription drugs were frightening. Proactive companies worried that one problematic material receipt could destroy their business.
Developing regulations that would effectively improve consumer quality, result in consistent products, and be implementable posed many challenges in this industry. The major problem for many manufacturers was to make a step-change from operating a company in an industry that had no required testing to one that now required a comprehensive quality control program. Although some products may be mixtures of few components, many have anywhere between twenty to thirty ingredients. Product proliferation is also rampant with formulations being designated for seniors, one for males, one for females, etc., resulting in a large number of SKU’s and thousands of different raw materials in the warehouse. This is especially true for contract manufacturers.
Then, there is the aspect of controlling the quality of natural products. Natural Vitamin C, for example, contains not only the nutrient identified as the vitamin, but also enzymes, coenzymes, antioxidants, and trace element activators. Phytomedicines are botanicals or herbal products derived from plants or parts of a plant valued for their therapeutic properties. Testing of phytomedicines and their raw materials is an extremely complex analytical problem. Different parts of the plant will have different composition such as root, leaf, flower or stem. The entire plant may be used in a formulation or a specific part of the plant. Botanicals may be in powdered or liquid form with or without a carrier. And, when raw botanicals are concentrated to form extracts, each individual process yields a different product, depending upon the process conditions. In order to reach a consistent level of actives, the extract is tested after production and “standardized” to the target value with native extract or with the corresponding synthetic material.
For example, an energy drink manufacturer may purchase powdered guarana extract according to a minimum level of caffeine as active on the specification. The producer of the extract would have to deal with natural variation of the incoming raw ingredient, and seek to achieve a target value for caffeine by adjusting process parameters such as optimum selection of solvents, the airflow rate, air inlet temperatures, and drying conditions. However, final testing of the extract may indicate that the caffeine level is lower than the minimum required amount. The manufacturer could then adjust the content of the active by adding synthetic or natural caffeine to the extract. The American Herbal Products Association (AHPA) has issued guidelines on the definition of an extract so that suppliers and manufacturers can use a common language when describing extract.
FDA Steps In
With all of these issues at play, the FDA developed the dietary supplement cGMPs so that manufacturers had some flexibility, provided their decisions were supported by good science and thorough documentation. Some examples will be covered later in this paper. The final rule is organized into 16 subparts which include requirements for designing and constructing physical plants; for establishing quality control procedures; for testing ingredients as well as finished products; for maintaining records; and for handling customer complaints.
In subpart A, The General Provision, it is interesting to note that the rule applies to companies or persons who “manufacture, package, label, or hold a dietary supplement” but does not apply to ingredient manufacturers or suppliers. Excluding raw dietary ingredient producers/suppliers from cGMP pushes the burden for ensuring quality of the supplement to the manufacturer of the final product. Quality is defined in Section 111.3 to mean that “the dietary supplement consistently meets the established specifications for identity, purity, strength, and composition and limits on contaminants and has been manufactured, packaged, labeled, and held under conditions to prevent adulteration.”
Overall, subpart B covers personnel requirements, how to establish and follow written procedures, and how to keep records; whereas subpart C outlines requirements for the physical plant and grounds to protect against contamination of components. Separate or defined areas of adequate size are needed to perform incoming raw material receipt, warehousing, production and testing.
Subparts D through P apply specifically to the manufacturing of dietary supplements and include specifics of quality control procedures, testing and record keeping. The cornerstone of this section is to establish written procedures. Section 111.30 requires that selected equipment is capable of operating within the limits specified by the process and must be routinely calibrated, inspected, or checked to ensure proper performance. Changes to operating parameters must be approved by quality control personnel and documented. From a quality control perspective, section 111.70 mandates that “you must establish specifications for any point, step or stage in the manufacturing process where control is necessary to ensure the quality of the dietary supplement,” and “you must establish component specifications that are necessary to ensure that the specifications for the purity, strength and composition of dietary supplements manufactured using the components are met.”
Limits of contamination that may adulterate or lead to adulteration of the final batch must also be established. Specifications for the packaging and labeling must also be established; including specifications that ensure that the specified packaging and labeling was applied to the current product. Section 111.75 requires that before the use of an incoming raw material or component “you must conduct at least one appropriate test or examination to verify the identity of that component.”
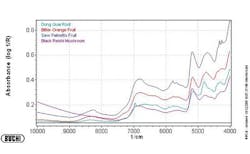
This minimum requirement to test identity has been one of the most daunting tasks manufacturers have faced in complying with this rule. Identity tests and specifications must be established for hundreds or thousands of natural and synthetic components used in production. Many companies have turned to NIR (near-infrared spectroscopy) as a rapid, non-destructive scanning tool to test incoming raw materials. The key to success with NIR is to properly implement the technology. Simply scanning raw material receipts from a supplier and establishing these spectra as “the reference” is not sufficient. This, in essence, is the same as not testing, but using the COA. The NIR technology must be trained with certified reference material, and/or received lots that have been independently tested using primary reference methods.
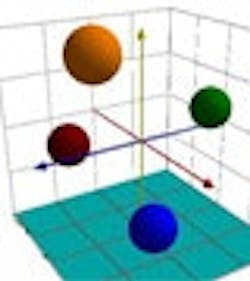
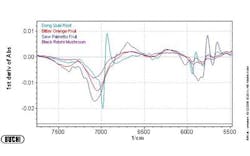
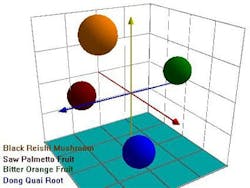
Figures 1-3 at the end of this article show, respectively, the NIR spectra of four nutraceuticals, NIR derivative spectra of the same products, and a graphic example of using a chemometric (principal components) calculation to distinguish between them.
Pure or simple materials can be analyzed in NIR by visual overlay and/or spectral correlation methods. However, spectral interpretation methods cannot always correlate to a complex matrix such as botanicals. There are too many variances within a complex matrix that do not allow for method specificity by visual interpretation or spectral correlation. Plants contain high amounts of cellulose which can easily mask any secondary metabolite differences that may be detected by spectral overlay. For this reason, spectral analysis by principal components methods have been successfully used to mathematically focus on these small differences in the presence of large absorbances. A database of “teaching” samples of certified reference materials and/or lots received that have been tested using primary reference methods define acceptable variation and unacceptable variation in a mathematical factor space. The software then assures that the acceptable tolerance limits of any particular material do not overlap with those of any other raw material.
Typically, at least five different, acceptable lots of material are used to establish tolerance limits, although additional lots are required for differentiation of materials that exhibit wide ranges of variation. Users group similar materials in the same principal component space, demonstrating specificity for each material. If materials overlap in principal component (PC) space, these materials would be deemed unsuitable for ID testing with NIR. In essence, the NIR method must be developed for each material to be tested and then validated for specificity and ruggedness. Dynamic updating allows new materials to be added in as needed through change control procedures.
Streamlining
Once properly implemented and validated, the NIR method allows for rapid ID as a material is received, streamlining the throughput. The regulation also allows for manufacturers to petition the FDA to request an exemption from 100% incoming raw material testing if sufficient evidence is provided that a supplier has been qualified. NIR can be useful in building a case to move towards reduced testing for selected materials and/or suppliers.
The last section in Subpart E discusses what manufacturers must do if established specifications are not met and governs sample retention and record retention requirements. Subparts F and G detail the Production and Process Control System including Requirements for Quality Control and Requirements for Components, Packaging, and Labels and for a Product received for Packaging or Labeling as a Dietary Supplement. The rule requires establishment of a Master Manufacturing Record as well as Batch Production Records. Subparts J, K and L are also part of the Production and Process Control System category, covering Requirements for Laboratory Operations, Manufacturing Operations and Packaging and Labeling Operations.
The Holding and Distributing of components, dietary supplements, packaging, and labels is governed in Subpart M. Under Subpart N, the rule requires that producers must establish and follow written procedures for compliance of Returned Dietary Supplements and that the returned materials be identified and quarantined until quality control personnel conduct a material review and reach a disposition decision.
Subpart O covers Product Complaints that must be reviewed. The indicated product must be investigated by a qualified person to determine if the complaint involves possible failure of the product to meet specification, with emphasis on evaluation of whether failing specification would increase the risk of injury or illness. Subpart P specifies what records must be made. Guidelines outline what records must be made available to the FDA.
Consumers have voted with their spending to make dietary supplements an integral part of their lives, as they shift their emphasis to preventative health care. According to the Natural Marketing Institute (NMI) 2007 Health & Wellness Trends Survey, the top health categories for supplements are weight loss, heart problems, digestion, arthritis or joint pain, seasonal allergies, vision and eye health and diabetes. At the same time, one of the key areas of consumer concern has been on counterfeit products. It is hoped that tighter control of the industry will assure patients of consistent purity and concentrations in their homeopathic medicines. Perhaps these regulations will lead to actual clinical safety, rather than merely depending upon apocryphal stories about the effectiveness of these products.