At Interphex 2008 in Philadelphia, David Radspinner, chairman of the ASTM E-5503 subcommittee on general pharmaceutical standards and marketing manager with Thermo Fisher Scientific, shared his views on Process Analytical Technologies (PAT) and Quality by Design (QbD).
In order for the industry to realize the full benefits of QbD and PAT, he said, it must change the way that it views its processes, even processes that seem as well established as drying. The key is moving from a “parametric” to a “functional” process description, which requires a different strategy on the regulatory, scientific and quality management sides.
This article will review cases that show how PAT, and methods including mass spectroscopy (MS), Near Infrared (NIR) spectroscopy, and differential scanning calorimetry (DSC), are being used to change the way that drug manufacturers describe the drying process and how they are using that information to better control and optimize that process. This is not meant to be an exhaustive or detailed analysis, but rather a summary.
In pharmaceutical manufacturing, minor fluctuations and deviations from an ideal path (at any step of a process) can cause the final product to fail final quality testing or worse, fail to perform in a patient. Under the present manufacturing paradigm, process operators have few, if any, checks to ensure that a process has run optimally. Instead, they must rely on older, inferential tests of such parameters as hardness, friability, and weight, and, finally, the QC release testing at operation’s end.
However, using PAT, various on-line instruments are used to monitor and automate a process. Using data gathered from any number of sources to model a “good” design space then allows us to predict and modify a batch that has drifted from an ideal design space. Traditional QA/QC lab techniques can then be used mainly for spot-checking and for calibration of the predictive method.
While the total control desired in pharmaceutical manufacturing is in its embryonic stage, there is no doubt that the FDA’s PAT initiative is spurring the increased use of numerous online analytics. Even the tentative use of these instruments is beginning to reveal the inner workings of the various unit operations with previously unseen levels of detail.
Case in Point: Bulk Material Drying
Since many pharmaceutical dosage forms begin with a wet granulation step or, at the very least, need to have a pre-determined level of moisture, a drying step is often included in a product formulation. One of the most time-consuming of any of the unit operations is the bulk drying of intermediates or active pharmaceutical ingredients (APIs), since large amounts of solvent and water must be removed from each wet-granulated product before it can move to the next process step.
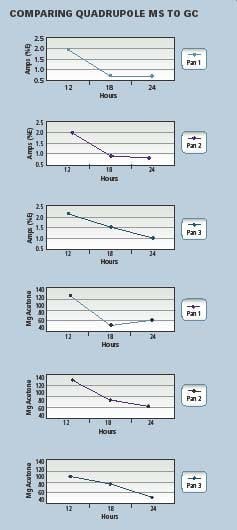
Fig 1. Correlation of GC and MS data
Vacuum dryers can accomplish the task, as can fluid-bed types or combination dryers which use both pressure and vacuum to facilitate drying and are often equipped with mechanical aids, such as stirring paddles or centrifuge action.
Without the benefit of online analytical data, this important production step has historically been performed by using a time-temperature-pressure recipe with periodic QA/QC testing to chart the progress of the process. There are variations to this approach, depending on the site and the product. However, the relationship between percent moisture or solvent and product performance has generally been viewed as straightforward. It often isn’t.
New methods are needed to answer such questions as: Where is the residual moisture? Was the effluent merely the desired solvent? Are we changing the physical and/or chemical properties of the materials involved during drying?
Some Manufacturing Examples
Example 1: Mass Spectrometry
Process mass spectrometry has been seen as an effective way to monitor and streamline drying. With multiple point sampling, and construction geared towards the industrial environment, it can achieve high sensitivity and selectivity when working with common pharmaceutical solvents. In fact, process MS can cut drying times by 50% or more, and can provide a wealth of information abut the drying process itself.
In one case, this analytical method was used to examine a drying process that was leading to off-spec coated tablets. Uneven product drying appeared to be occurring. Operators at the company suspected that this occurred because airflow might have been stratified as it ventilated the trays.
A commercial process mass spectrometer [1] was used to determine whether there are variations in the bed temperatures, in addition to allowing the production personnel to evaluate the process itself.
This particular dryer was equipped with eight sampling points: one each in the dryer inlet and exhaust plenums and six points in the dryer volume separated equidistantly from top to bottom and front to back in the dryer. (This sampling arrangement provides a spatially differentiated look at each dryer along with totalized dryer input and output solvent levels by using the inlet and outlet sample points.)
The analysis was performed by stepping the mass spectrometer through each sample point in each dryer in a programmed sequence. Results suggested that stratification was occurring, allowing the company to respond.
What Type of Results May Be Seen
A mass spectrometer provides a simple picture of the work being done by the dryer during different phases of the drying cycle. In the case of, say, aspirin — either pure or in a formulation — not only drying, but API stability may be monitored, as well as moisture and whichever organic solvent may have been used. The mass spectrometer is capable of following generation of acetic acid, for example, which would immediately show that the aspirin was degrading and that drying conditions needed to be improved. Corrective action could thus be taken before the batch was ruined.
Measuring variations in the drying rates between points may well show the variations in air flow throughout the drying container.
This will lead to SOP changes in the manner in which the dryer is charged and/or operated. Modifications to air flow and temperature as well as load capacity, based on real-time experimental results, are an integral step along the road to PAT and control. Figure 1 (at right) shows some actual data from a commercial run, comparing conventional gas chromatography with the MS data. The time ratio alone makes MS worth performing in this case.
Example 2: Thermal Analysis
Several years ago, some investigational work was being performed on an API, in this case, a morphine derivative, using differential scanning calorimetry (DSC). The research sought causalities between the active ingredient and variations observed in stability data.
Although no differences in drug solubility or dissolution were apparent, some lots of the API seemed to fail stability up to six months before the others. The degree of drying, after granulation, was measured by the total moisture content of the granulation, which fell from nearly 20% to 1% at endpoint. The first three commercial level batches were dried to 1% and that was the “timed” endpoint for all subsequent lots.
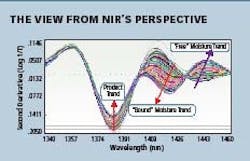
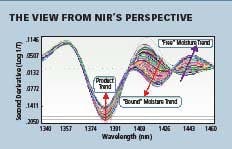
Fig 2. NIR spectra of a granulation during the drying process
However, using DSC, a researcher at the company discovered that, depending on lot size, air flow, and drying temperature (it was a fluid bed dryer), either a monohydrate or a hemi-hydrate could form. Both forms were extremely soluble, so there was never a problem with blood levels or release parameters. The difficulty arose when stability samples were run. It appears that form A was less stable than form B and had a six-month shorter shelf life.
This project applied the rudimentary beginnings of QbD, wherein the product and everything about it is examined, both physically and chemically. By applying a knowledge-based decision instead of 1950s process parameters, the company saved itself the expense of numerous recalls over the product’s lifetime.
Example 3a: Near-Infrared
Another drug company was interested in where the water actually resided upon the drying of a granulation. Previously, an LOD or Karl Fischer analysis was considered sufficient, giving hard numbers such as 1.5% or 2.2%. Now, someone wanted to know more about the nature and location of the water.
It was deemed that NIR, which is quite good at moisture measurements, might answer more questions than simply “how much?” NIR spectrometers, in general, are full spectrum instruments and contain a plethora of information about the chemical and physical nature of complex mixtures. The chemometrics packages that are an integral part of NIR are ideal for discriminating between minute variations in consistency and composition.
Several drying processes were monitored by NIR, with samples being taken for other lab-based analyses. It was seen that the “bound” or crystalline water, attached to the product had different spectral characteristics from “free” or surface water in the same mixture. Figure 2 (above right) shows NIR spectra of two water bands: an increasing “bound” and a decreasing “free.”
By observing just a few wavelengths, production analysts were able to determine the ideal conditions to produce the desired ratio of free water to bound water. The rates observed also allowed them to optimize the airflow and temperature for the process. This “process signature” they developed also allowed for varying sized batches to be produced.
Example 3b: Near Infrared
In another study, an instrument manufacturer [2] monitored several dryers to show how closely they behaved. While the original purpose was to show that all these dryers could be used interchangeably, the research offered side benefits, demonstrating that product drying times could be dramatically shortened and showing areas where maintenance needed to be improved.
As seen in a number of other studies, performed by numerous suppliers of equipment, the time to dryness was often much shorter than the time specified in the standard operating procedure (SOP). In one such case, the limit of less than 5% was reached many hours before the time specified in the master manufacturing form (MMF).
An added benefit was uncovered when NIR was used to compare several of the “same” dryers. The analyses found that the three did not behave like each other. Upon investigation, the differences in performance were traced to maintenance issues: a clogged drying sock in one case, a leaking O-ring in another. All dryers were reaching the correct endpoint in the “correct” time, but the NIR showed how two of them could be corrected to do better. The time saved through work like this can prevent a company from having to purchase, install, house, and validate more dryers. In this case, the efforts allowed existing equipment to be made many times more efficient.
Practical, Not Arcane
It is critical to remember that PAT-type measurements are not the stuff of science fiction. They are “now,” and these are but a few examples of how tools for PAT are being used on pieces of the total process, with great success. PAT is not just hardware; it is not just data and process changes when problems are found. It is an entire paradigm shift: from “hunt and peck” production troubleshooting to actually understanding the process, and using this knowledge to make it trouble-free.
Product and the manufacturing equipment itself may be improved using modern analysis techniques. A company needn’t make a multi-million dollar (or Euro) commitment for PAT to begin. There is so much “wiggle room” in current pharmaceutical production practices that even a small step — a mediocre attempt at PAT — may yield handsome dividends. Can you imagine the rewards for getting it perfect? And all this success was merely in the drying step.
References:
1. Ametek Promaxion
2. ABB Bomem