Improving Control of the Extrusion Spheronization Process
Pellets are increasingly being used as multiple unit-dosage forms. They offer manufacturing advantages such as improved flow, reduced friability, narrow particle size distribution, ease of coating and uniform packing, but they also offer therapeutic benefits. For instance, they prevent the buildup of high local concentrations of bioactive agents that might irritate the stomach. They also reduce the variability of plasma profiles between subjects and within the same patient, by reducing variations in gastric emptying rates and overall transit times.
Pharmaceutical pellets are typically manufactured via extrusion spheronization, a three-step process introduced in the late 1960s, that results in spherical granulates roughly 1 mm in diameter. In this process, the powder is formed into a wet mass, which is forced through a restricted area (extrusion) to form strands of extrudate that are broken into short lengths and rounded by placement on a rotating plate within a cylinder.
The resulting spherical granules or pellets are of uniform shape, size and density. When dry, the spheroids have an extremely low friability and are ideally suited for film coating.
Extrusion spheronization’s major advantage over other methods of producing drug-loaded spheres or pellets is its ability to incorporate high levels of active components without producing an excessively large particle.
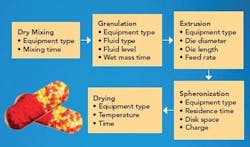
The process requires at least five unit operations, with an optional sixth screening step. Figure 1 shows each of the process steps along with the critical variables associated with them.
Figure 1. Process flow chart of the extrusion spheronization process, showing the process variables for each individual step.
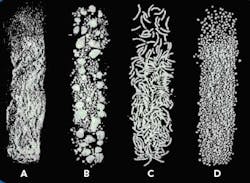
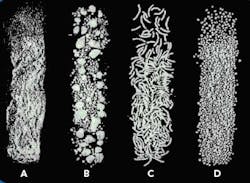
The end product from each of the steps is shown in Figure 2.
Figure 2. Product produced by the first four extrusion spheronization process steps: (a) powder from dry mixing, (b) granules from granulation, (c) extrudate from extrusion, and (d) spheres from spheronization.
During the first step, powders are dry-mixed to achieve a uniform dispersion before wet granulation. It is generally carried out in the same mixer used for the granulation; however, if a continuous granulator is used, a separate mixer is required for the dry mix. The second step is granulation, during which a wet mass, having the requisite plasticity or deformation characteristics, is prepared. Granulation can be done by batch-type processors (which include planetary mixers, vertical or horizontal high-shear mixers), sigma-blade mixers or continuous mixers (which include the Nica M6 instant mixer6 and high-shear twin-screw mixer-extruders7).
The major difference in this process granulation step is that the amount of fluid needed to achieve spheres of uniform size and sphericity is likely to be greater than that for a similar granulation intended for tableting. This is important to achieving uniform fluid dispersion.
Extrusion is the third step of the process; it consists of shaping the wet mass into long rods, commonly termed ‘extrudate.’ The extrusion process is currently used as an alternative method for the manufacture of completely water-soluble tablets. Extruders come in many varieties, but can generally be divided into three classes.
Screw Feed Extruders
The primary difference between types of screw extruders is in the extrusion zone. An axial or dome extruder transports and extrudes the wet mass in the same plane. Various types of screw-feed extruders are listed below.
- Axial extruders force the wet mass through a flat, perforated end-plate, prepared by drilling holes in a plate.
- Dome extruders use a dome or half-sphere-shaped screen as the die.
- Radial extruders transport material into the extrusion zone where tapered extrusion blades wipe material through a perforated screen.
Gravity Feed Extruders
Gravity-feed extruders include cylinder, gear and radial types. The cylinder and gear types both belong to a broader class, referred to as roll extruders. Both use two rollers to exert force on the wet mass and form an extrudate.
- Cylinder extruders have rollers in the form of cylinders: one solid and one hollow, with drilled holes to form the dies. The wet mass is fed by gravity into the nip area between the two cylinders and forced through the dies into the hollow of the cylinder.
- Gear-type extruders have rollers in the form of hollow gears. The dies are holes drilled at the base of each tooth. Wet mass is forced through the holes and collected in the hollow of the gears as the teeth and the base areas mesh.
- Radial extruders involve rotation of one or more arms to stir the wet mass as it is fed by gravity. Rotating blades wipe the mass against the screen, creating localized forces sufficient to extrude at the nip.
Ram Extruders
Ram extruders consist of a chrome-plated barrel positioned in a thermostatically controlled storage tunnel. A range of dies can be fitted to the extruder head. Material is loaded into the barrel manually or mechanically and vacuum is applied to eliminate air from the system. The material is extruded through a hydraulically powered ram, with the hydraulic fluid (oil) being passed through a special valve system to sense changes in the plasticity of the material and compensate ram pressure to achieve an even extrusion through the hole.
Spheronization
The working parts consist of a bowl having fixed sidewalls, with a rapidly rotating bottom plate or disk. The rounding of the extrudate into spheres is dependent on frictional forces. Accordingly, the disk is generally machined to have a grooved surface, which increases the forces generated as particles move across its surface. Disks having two geometric patterns are produced: a cross-hatched pattern, with the grooves running at right angles to one another, and a radial pattern, with the grooves running outward from the center. Some studies have shown the rate of spheronization to be faster with the radial pattern; however, either plate will result in an acceptable product.
Mechanisms of Sphere Formation
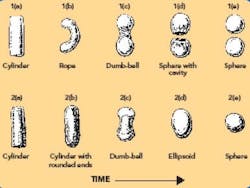
The transformation from cylinder-shaped extrudate to a sphere occurs in various stages. Two models have been proposed to describe the mechanism (shown graphically in Figure 7).
Figure 7. Two mechanisms of spheronization.
The model proposed by Rowe8 describes a transition whereby the cylindrical particles (see Fig. 7-2a) are first rounded off into cylindrical particles with rounded edges (see 7-2b), then form dumbbell-shaped particles (see 7-2c), ellipsoids (see 7-2d), and finally, spheres (see 7-2e). The second model, proposed by Baert et al.,22 suggests that the initial cylindrical particles (see 7-1a) are deformed into a bent, rope-shaped particle (see 7-1b), then form a dumbbell with a twisted middle (see7-1c). The twisting action eventually causes the dumbbell to break into two spherical particles, with a flat side having a hollow cavity (see 7-1d). Continued action in the spheronizer causes the particles to round off into spheres (see 7-1e). When the sphere is fractured, a hollow particle is revealed. The exact mechanism is likely composition-dependent.
Spheronization is a process invented in 1964 by Nakahara. The patent describes a method and apparatus for making spherical granules from wet powder mixtures.The equipment described in the patent was commercialized by Fuji Denki Kogyo Co. under the trade name ‘Marumerizer.’ The process went widely unnoticed in the pharmaceutical industry until 1970, when two articles were published by employees of Eli Lilly & Co. Conine and Hadley described the steps involved in the process, including(a)dry blending, Reynolds went on to further describe the equipment and the mechanics of the process, including the movement of the particles within the spheronizer. |
Batch and Continuous Operation of Spheronizer
Two systems have been developed to enable the extruder to continuously feed material to the spheronizer(s):
- The shuttle system uses two spheronizers in parallel. It is designed to fill one spheronizer while the second is in the middle of its cycle, continue to collect extrudate in a shuttle receptacle while both are full and operational, and fill the second after it empties and the first unit is in the middle of its cycle.
- The cascade system uses one or more spheronizers9. The product is continually fed from either the extruder or a previous spheronizer; as the charge volume grows from incoming material, some product is discharged. The number of spheronizers placed in sequence depends on the desired outcome.
Drying
Drying is the final step in the process. This can be accomplished in any dryer that can be used for conventional-type granulations, including tray dryers, column-type fluid beds and deck-type vibratory fluid beds.
Process Parameters
The process of extrusion and spheronization is a multi-step one involving a number of parameters that have a bearing on the final characteristics of the obtained pellets. If moisture content is above a certain upper limit, then an overweighed mass and agglomeration of individual pellets during spheronization results because of an excess of water at the surface of pellet10. The moisture content also influences the mechanical strength, friability, internal porosity and the particle-size distribution of pellets.
Ostuka et al.11 reported that the internal porosity of spherical granules decreases with increasing water concentration; weight loss after the friability test increases with a decreasing amount of water and the water volume influences the mechanical strength of the granules. Moisture content also affects the shape and size of granules12. Gazzaniga et al.13 found differences in the friability and particle size of pellets when the powder mass was wetted with different quantities of water.
Starting Material
The physical nature of the starting material influences the particle size, hardness and sphericity, as well as the release rate of the included drug. There is not only the obvious difference in pellet quality produced from different compositions, but also a difference when various forms of the same product are used14 The use of products that are similar but from different suppliers has also been found to change the characteristics of the pellet.15, 16
Pellets prepared with three types of microcrystalline cellulose (MCC) from different manufacturers featured differences in size and roundness when processed under the same conditions15. The physical properties of two types of commercial MCC showed differences during the step of moistening, thereby affecting the particle size and hardness of the pellets obtained17.
Granulation Liquid
The most commonly used granulating liquid is water, although in some cases the use of alcohol or a water-alcohol mixture has also been reported18. The effect of the alcohol content in a water-alcohol mixture has been extensively studied by Millili and Schwartz19.
Differences in friability and dissolution were observed between pellets that were water-granulated vs. those granulated with a mixture of 95% ethyl alcohol in water. Increasing the water content of the granulation liquid leads to an increase in hardness of the pellets. Promising preliminary results were obtained by lowering the solubility of β -CD in the wetting liquid through the use of water-alcohol mixtures. This probably improves the plasticity of the wetted mass and thus the feasibility of the overall process.
Extruders
According to Reynolds20 and Rowe21 an axial screw extruder produces a denser material than a radial screw extruder. The latter has a higher output but also produces a greater rise in the temperature of the mass during processing.
Pellet quality is dependent on the thickness of the screen and the diameter of the perforations.
Baert et al22 reported differences in extrudate quality when they were obtained by extrusion with screens of different thicknesses. A thinner screen formed a rough and loosely bound extrudate, whereas a thicker screen formed smooth and well-bound extrudate because of the higher densification of the wet mass. Similarly, the diameter of the perforations determines the size of pellets — a larger diameter in the perforations will produce pellets with a larger diameter when processed under the same conditions23, 24.
Extrusion Speed
The total output of the extruder is mainly governed by the extrusion speed. Several studies show that surface impairments, such as roughness and shark-skinning, become more pronounced with increasing speed23, 24.
Surface impairment of extrudate leads to pellets of lower quality because the extrudate will break up unevenly during the initial stages of the spheronization process, resulting in a number of fines and a wide particle-size distribution25
Extrusion Temperature
A rise in temperature during the extrusion cycle could cause some of the liquid to evaporate from the granules. In turn, that may lead to a difference in the quality of the extrudate produced at the beginning of the batch vs. the end. Extrusion temperature control is especially important when processing a thermolabile drug formulation.
Spheronizer Specifications
Pellet quality is also dependent on spheronizer load. It mainly affects the particle-size distribution, bulk and tap density of the final pellets18 The yield of pellets of a specific range decreases with an increase in the spheronizer speed and a low spheronizer load, whereas it increases with extended spheronization time at a higher spheronizer load26, 27. Barrau et al.27 reported that an increasing spheronizer load decreased the roundness and increased the hardness of pellets, whereas yield in the majority size range remained unchanged. Hellen et al.28 reported that the bulk and tap density increased and the size of the pellets decreased with an increasing spheronizer load.
The hardness, roundness, bulk and tap density, porosity, friability, flow rate and surface structure of pellets are also affected by a change in the spheronization speed. Spheronization time mainly affects the particle-size distribution and the bulk and tap density of the pellets29.
Advantages of Extrusion and Spheronization
- Ease of operation
- High throughput with low wastage
- Narrower particle-size distribution
- Production of pellets with low friability
- Production of pellets that are suited for film coating
- More sustained, better controlled drug-release profile
- Uniform size distribution
- Ease of coating
- Reproducible packing
- Good flow
- Low dusting.
Potential pharmaceutical applications are many, including both immediate- and controlled-release dosage forms. Two or more active pharmaceutical ingredients (API) can easily be combined in any ratio in the same unit. These combination products can contain active agents that are incompatible or have varying release profiles. Spheres can be used as a method to limit drug migration. Physical characteristics of the active ingredients and excipients can be modified to improve physical properties and downstream processing.
Products produced by using extrusion spheronization can range from barely shaped, irregular particles with physical properties similar to a conventional granulation, to very spherical particles having properties that are drastically different.
The process of wet extrusion, followed by spheronization, is used to produce a wide variety of engineered, controlled-release drugs. These are mostly tablets or capsules containing high levels of an API.
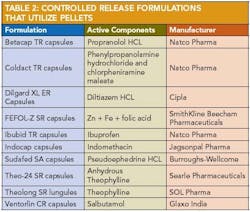
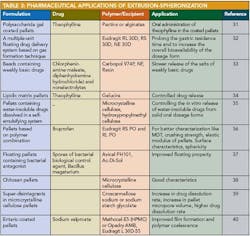
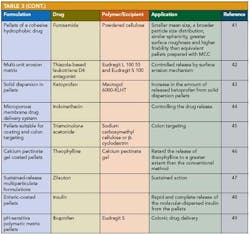
Applications of pelletization in controlled-release formulations and pharmaceutical applications of extrusion spheronization 30 are shown in Table 2 and Table 3.
Conclusions
Extrusion spheronization is the only technology that provides improved spheronization and pellets with desired characteristics. It is suitable not only for the manufacture of pellets with a high drug-load, but also for producing extended-release pellets in the same step in certain situations. Although various process parameters may affect pellet quality, the process creates pellets with a desired drug-release profile when a controlled formulation procedure is followed.
Extrusion spheronization is an effective technique for the preparation of controlled-release multi-particulate formulations of bioactive agents. In addition, the process is capable of high throughput with low wastage and easy operation. It is envisaged that extrusion spheronization will become a popular process in the near future.
About the Authors
All five co-authors are affiliated with the S.K. Patel College of Pharmaceutical Education and Research, Ganpat University, Kherva, Gujarat, India. Rakesh P. Patel is an Assistant Professor in the Dept. of Pharmaceutics and Pharmaceutical Technology. Dr. Jayvadan K. Patel is an Assistant Professor in the Dept. of Pharmaceutics and Pharmaceutical Technology. Dr. Madhabhai M. Patel is Principal and Head of the Dept. of Pharmaceutics and Pharmaceutical Technology. Nikunjana A. Patel is a lecturer in the Dept. of Pharmacognosy. Darshana M. Patel is doing postgraduate study in Pharmaceutics.
References
- Ghebre-Sellassie I., Pharmaceutical Pelletization Technology, Marcel Dekker, Inc., New York, 1989; 1-13.
- Nakahara. U.S. Patent 3, October 1966, 277-520.
- Conine J.W., Hadley H.R., Preparation of small solid pharmaceutical spheres; Drug Cosm Ind. 1970; 106:38-41.
- Reynolds A.D. A new technique for the production of spherical particles; Manuf Chem Aerosol News. 1970; 41: 40-43.
- O’Connor R.E., Holinez J., Schwartz J.B. Spheronization I: Processing and evaluation of spheres prepared from commercially available excipients. Am J Pharm 1984; 156: 80-87.
- Hellen L., Yliruusi J., Merkku P., Kristoffersson E. Process variables of instant granulator and spheronizer. Int J Pharm. 1993; 96: 197-204.
- Kleinebudde P., Lindner H. Experiments with a twin screw extruder using a single-step granulation/extrusion process. Int J Pharm 1993; 94:49-58.
- Rowe R.C. Spheronization: a novel pill-making process; Pharm Int 1985; 6:119-123.
- Hicks D.C., Freese H.L. Extrusion and spheronization equipment. In: I Ghebre-Sellassie, Ed. Pharmaceutical Pelletization Technology. New York: Marcel Dekker, 1989, 71-100.
- Fielden KE., Newton JM., Rowe RC. The influence of lactose particle size on spheronization of extrudate processed by a ram extruder. Int J Pharm. 1992; 81: 222–233.
- Ostuka AM., Gao J., Matsuda Y. Effect of amount of added water during extrusion-spheronization process of pharmaceutical properties of granules. Drug Dev Ind Pharm. 1994; 20: 2977–2992.
- Fielden KE., Newton JM., Rowe RC. The influence of moisture content on spheronization of extrudate processed by a ram extruder. Int J Pharm. 1993; 97: 79–92.
- Gazzaniga A., Bruni G. The use of β - cyclodextrin as a pelletization process agent in the extrusion/spheronization. Drug Dev Ind Pharm. 1998; 24: 869–873.
- O’Connor RE., Schwartz JB. Spheronization: II. Drug release from drug-diluent mixtures.Drug Dev Ind Pharm. 1985; 11: 1837–1857.
- Newton JM., Chow AK., Jeewa KB. The effect of excipient source on spherical granules made by extrusion/spheronization. Pharm.Tech. Int. Biophys. 1992; 4: 52-58.
- Law MFL., Deasy DB., Mclaughlin JP. J Microencap. 1997; 14: 713–723.
- Sonagllo D., et al. Drug Dev Ind Pharm. 1995; 21: 533–547.
- Vervaet C., Baert L., Remon JP. Extrusion-spheronization: a literature review. Int J Pharm. 1995; 116: 131–146.
- Millili GP., Schwartz JB. The strength of microcrystalline cellulose pellets: The effect of granulating with water ethanol mixtures. Drug Dev Ind Pharm. 1990; 16: 1411–1426.
- Reynolds AD. A new technique for the production of spherical granules. Manuf. Chem. Aerosol News. 1970; 41: 40–43.
- Rowe RC. Spheronization: A Novel Pill-Making. Pharm Int. 1985; 5: 119–123.
- Baert L., Remon JP., Elbers JAC., Van Bommel EMG. Comparison between a gravity feed extruder and a twin screw extruder. Int J Pharm. 1993; 99: 7–12.
- Hellen L., Yliruusi J., Muttonen E., Kristoffersson E. Process variable of the radial screen extruder: II. Size and size distributions of pellets. Pharm.Tech. Int. Biophys. 1993; 5: 44–53.
- Harrison PJ., Newton JM., Rowe RC. Flow defects in wet powder mass extrusion. J Pharm Pharmacol 1985; 37: 81–83.