Using MEMS to Control Blending at AstraZeneca
Blending of active pharmaceutical ingredients (APIs) with various excipients is a common step in the pharmaceutical solid dosage form manufacturing process. The homogeneity of the blend is critical in defining the uniformity of dosage units within a batch of tablets, especially in the case of direct compression products.
Typically, the API in the compressed blend is analyzed in the finished dosage form for potency and content uniformity. For many existing formulations, it is important to ensure that both the active and excipients are uniformly distributed throughout the solid dosage form. It is important to note that each pharmaceutical product has a distinctive blend of API and excipients and thus each blending operation is also unique.
The generic pharmaceutical industry implemented wide spread blend testing as a response to Judge Wolins opinion in the United States vs. Barr Laboratories court case in 1993 (Civil Action No. 92-1744). It was stated by Judge Wolin that blend testing is necessary to increase the likelihood of detecting inferior batches. Blend content uniformity testing cannot be waived in favor of total reliance on finished product testing, because finished product testing is limited. It was therefore concluded that final blend testing was required for each batch of a drug product, including ordinary production batches.
Additionally, blend uniformity is addressed in the Current Good Manufacturing Practices (cGMP) regulations and drug approval programs. Section 211.110 of cGMP requires manufacturers to establish control procedures (that) include adequacy of mixing to assure uniformity and homogeneity. The regulations, however, did not specify the blend testing approach, nor the particulars as to the acceptance criteria, limits, or methods for the testing. Once again in 1996, the FDA implied the need for blend uniformity testing of all routine manufacturing batches in an effort to make certain that a high level of quality was maintained throughout a process, and not just upon completion of the final product. (1)
In order to provide a response to the FDA and in an attempt to produce a relevant guidance, the Blend Uniformity Working Group (BUWG) was formed under the auspices of the Product Quality Research Institute (PQRI). After several revisions, BUWG developed a draft recommendation on stratified sampling of powder blends that was presented to the CDER in December 2002 and published in 2003. (2) Based upon these recommendations, the FDA published their draft guidance on stratified sampling in October of 2003. (3) The report and the new draft guidance describe the use of a stratified sampling scheme for demonstrating blend uniformity based upon thieving multiple samples from the blender in multiple places and analyzing the samples for blend uniformity via the appropriate reference method.
The accepted technique used for the sampling of powder from blenders is thief sampling. The main drawback of thief sampling is that the technique is difficult to reproduce and often the act of sampling may cause sample inhomogeneity and lead to result bias. Other potential issues with thief sampling are that it is labor intensive for both the operator and laboratory personnel and there is often a long delay from the time the blender is stopped to the time the blend uniformity result is received. There is also the concern for operator exposure, especially in the cases of high-potency APIs, and the operators must be fully gowned in order to grab samples. (4)
One potential solution to satisfy the need for blend sampling while avoiding the issues with thief sampling is the on-line monitoring of powder blending by near infrared (NIR) spectroscopy. In September 2004, the FDA published the draft guidance, PAT A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Control, which opened the door for manufacturers to explore implementing on-line techniques for real-time blend uniformity analysis. (5)
The goal of the FDA is to encourage pharmaceutical manufacturers to build quality into products through process control and understanding rather than testing quality at the finished product stage. NIR spectroscopy is one of the techniques ideally suited for PAT applications, and has gained acceptance within the pharmaceutical industry and regulatory agencies.
NIR spectroscopy can be used to collect high quality reflectance spectra of both the active ingredient and excipients and is sensitive to chemical as well as physical properties of the powder blend. Advantages to NIR spectroscopy include that it is noncontact and nondestructive, highly reproducible, rapid and requires no sample preparation. On-line blend monitoring places certain demands on NIR instrumentation including wireless communication, battery operation, rapid data collection, appropriate hazard and cleaning rating and software/hardware validation and qualification.
It is also important that the instruments can be used from pilot- to production-scale blenders in order to follow the development of a pharmaceutical product. NIR spectroscopy has previously been used for on-line blend monitoring using instruments based on diode array and acousto-optical tunable filter technologies. (6) This paper discusses the application of a MEMS-based NIR spectrometer for on-line monitoring of powder blending in a rotating bin blender.
MEMS is an acronym for Micro Electro Mechanical Systems and are tiny, micro-fabricated devices that can be found in a variety of industries including defense (munition guidance, surveillance), automotive (tire pressure, air bag sensors), electronics (projection screen TVs, ink jet printing heads, video game controllers) to name a few. MEMS is an expansion of the tools and manufacturing methods existing in the microelectronics industry and, unlike typical integrated circuits, these devices will have some non-electronic function as well such as sensing, communication or actuation.
MEMS application to spectroscopy means that the now-bulky optical/mechanical part of a spectrometer can be miniaturized to match the microelectronic solutions that are currently available. This combination produces a combined microoptic-micromechanical-microelectronic system. Advantages of MEMS-based NIR spectrometers are their small size and weight, and, since these spectrometers can be machine-made and mass-produced, the resulting instruments are nearly identical, have 20+ lifetimes, and, eventually, will become less expensive. (7) Recently, MEMS-based systems have been used in the pharmaceutical and chemical industries for raw material identification, solvent recovery and dryer control and monitoring (8).
Experimental
The instrumentation used for this work is an Antaris Target blend analyzer (Thermo Electron Corp.). This device is a MEMS-based NIR analyzer with a spectral range of 1350 1800 nm. The spectrometer is configured with a semiconductor-based NIR tunable laser source, high-resolution (1.0 nm optical resolution) Fabry-Perot tunable filter for wavelength selection, and single element InGaAs photodiode detector.
The spectrometer bench is contained, under dry nitrogen, within a hermetically sealed compartment. Each spectrum produced from the spectrometer is referenced to an internal standard ensuring optimal wavelength and absorbance reproducibility. The unit is battery-powered and uses a MEMS-based accelerometer board to determine blender position and thus initiation of data collection. This instrument is capable of scan speeds of approximately 10 scans per second.
The spectrometer has no moving parts and scan performance is insensitive to both blender position and vibration. It can easily be attached to a modified blender lid and collects NIR spectra through a sapphire window built into the blender lid. Data is collected when the material in the blender is against the window at the bottom part of the blenders rotation. A spot size of 40mm was used corresponding to a 600mg dosage form.
Results
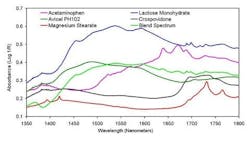
The blender used for these studies is a Bohle bin blender making use of Bohles patented counter-current blending technology. The initial testing of this instrument was accomplished using a model formulation of acetaminophen (APAP), Avicel PH102, lactose monohydrate, crospovidone and magnesium stearate. The static MEMS collected absorbance spectra of the active (acetaminophen), excipients and a blended sample are shown in Figure 4. Notice that the blend spectrum is a composite of the active and excipient spectra.
The model active and excipients were then loaded into a 20 liter bin blender with a modified lid, and the Antaris Target blend analyzer was attached to the blender. Data collection was configured so that five scans of the powder blend were collected and averaged during each blender revolution providing a single spectrum for each rotation. Data collection was initiated by the MEMS accelerometer board and programmed to begin when the blender was at approximately a 160º rotation. The spectral data collection was completed in approximately 500 ms.
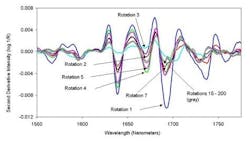
Example spectra collected during the blend have been plotted in Figure 5. Note the spectra in Figure 5 have been mathematically pretreated via calculation of the second derivative spectrum. Calculation of the second derivative spectrum has the effect of minimizing spectral baseline differences while enhancing subtle spectral features. A large degree of spectral variation is noticed early on in the blending process and then is reduced greatly after rotation 15.
Since the NIR spectra reflect the chemical properties of the powder in the blending vessel, it can be argued that as the variation in the collected spectra is reduced, the mixture in the blender is becoming more uniform. Thus, when the spectral variation reaches a minimum, the blended product has reached its blend endpoint. There are many potential mathematical algorithms available for the determination of blend endpoint. (9)
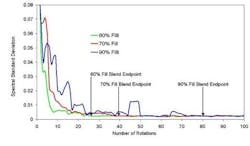
One such method to determine a numerical value for the blend endpoint is by calculating a moving block standard deviation of the spectra. (10) This is done by first selecting a spectral region of interest. This can be a spectral region where only the active or component of interest has an absorption peak, or a region that is inclusive of all components. Next, a block size of rotations to group together is selected. A block size of seven was selected for this model blend. Following these selections, the variance and standard deviation at each wavelength and for each data block can be calculated. The standard deviations are then summed for each block across all wavelengths providing a single sum total standard deviation for each block. The summed standard deviation can then be plotted versus the number of rotations thus converting the spectral data into easy to interpret blending plots as shown in Figure 6.
Figure 6 illustrates the results from blending the selected model blend using a 20-liter bin blender and varying the amount of material to produce a 60%, 70% and 90% filled bin. One can clearly see the importance of blend endpoint determination via NIR as the bin that was 90% filled required approximately three times the number of rotations as the 60% filled bin. This effect may also be seen as new excipient lots are brought into processing and properties such as bulk density, moisture content and surface areas are changing. This plot shows the importance of spectral-based endpoint determination rather than time-based process control monitoring.
Conclusion
The MEMS-based, NIR Antaris Target blend analyzer can be used as an on-line, real-time, blend monitoring and control tool for determination of blend end-point and conformation of blend uniformity. A variety of parameters within the blending process may have an impact on blend uniformity and an example was shown where an increased fill level of the bin blender led to blend times that were much longer than expected.
Other parameters that will be studied in regard to blend endpoint determination and control are active concentration, blender size, blender speed, and active/excipient loading methods. The effect of scale-up from pilot scale to manufacturing will also be followed using the spectral endpoint determination methods.
Research into algorithms for the determination of blend endpoint will also be investigated to determine the most appropriated algorithm for a given product. Finally, after assuring the uniformity of the blend, the long term goal will be to follow the blended material throughout the process confirming uniformity of the blend at key processing steps including just prior to tablet compression. After compression, the uncoated tablets could be analyzed for content uniformity thus confirming product quality and potentially eliminating the need for further laboratory blend or tablet uniformity testing.
FOOTNOTES
- FDA, Current Good Manufacturing Practice: Amendment of Certain Requirements for Finished Pharmaceuticals; Proposed Rule, (61 FR 20103) May 1996.
- Boehm G, Clark J, Dietrick J, Foust L, Garcia T., Gavini M., Gelber L., Goeffroy J.M., Jimenez P., Mergen G., Muzzio F., Planchard J., Prescott J., Timmermans J. and Takiar N., The Use of Stratified Sampling of Blend and Dosage Units to Demonstrate Adequacy of Mix for Powder Blends, PDS J Pharm Sci Tech 57, pp. 59 74, 2003.
- FDA, Guidance for Industry: Powder Blends and Finished Dosage Units Stratified In-Process Dosage Unit Sampling and Assessment, October 2003.
- Hwang, Ruey-Ching and Wu, Sy-Juen, Challenges of Blend Uniformity Testing for Tablet Formulation, Am. Pharm Rev 7, pp. 101 103, January/February 2004.
- FDA, Guidance for Industry: PAT A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance, September 2004.
- Cogdill R., Anderson C., Delgado-Lopez M., Molseed D., Chisholm R., Bolton R., Herkert T., Afnan A., and Drennen III J., Process Analytical Technology Case Study Part I: Feasibility Studies for Quantitative Near-Infrared Method Development, AAPS Pharm Sci Tech 6 (2), pp. E262 E272, 2005.
- Crocombe R., MEMS Technology Moves Process Spectroscopy into a New Dimension, Spectroscopy Europe, pp. 15 19, July 2004.
- Parris J., Airiau C., Escott R., Rydzak J. and Crocombe R., Monitoring API Drying Operations with NIR, Spectroscopy 20(2), pp. 34 42, February 2005.
- Sullivan M., The Use of NIR as a PAT Tool for Measuring Blend Uniformity, Spectroscopy xx(x), pp. 1-4, March 2005.
- Selkulic S.S., Wakeman J., Doherty P. and Haily P.A. Automated System for the On-line Monitoring of Powder Blend Processes Using Near-Infrared Spectroscopy Part II: Qualitative Approaches to Blend Evaluation, J. Pharm Biomed Anal. 17, pp. 1285 1309, 1998.