This is the third year that the conference has been within shouting distance of the FDAs sites in the Washington, D.C., area. The proximity and involvement of FDA personnel have greatly impacted the program (and I could drive from New York and save a few Euros). There were sessions often three, four or five happening simultaneously, every day. I cannot remember a meeting where I was so stretched. Many other conferences, it is difficult to find ANY session worth attending, let alone several. I will attempt to break down the proceedings into logical packets. But there were so many things to see
Presentations
It was an exercise is being in six rooms at a time to try to see all the presentations I wanted to attend. There were, of course, basic What is PAT? talks for the beginners in the crowd to go with some pretty detailed talks for those well into PAT.
The Tuesday plenary sessions were a mix of basic primer and meat. All industries were included, but the strong presence of the FDA was apparent. Jeff Gunnell and Bill Floyd represented ExxonMobil quite well. And it is apparent that petrochemicals (as well as most other industries) are well ahead of pharmaceutical companies when it comes to process control. While it might be said that potato chips and gasoline are not potent drugs (except that our President [Carter, wasnt it?] said we are addicted to oil), we recognize that more than caution is involved.
Tim Moore (Genentech) had some pretty salient comments on biotech manufacturing; however, much of the talk was on what they will be doing. Several talks were included on Homeland Security (Laura Skubal, Argonne National Lab and Quentin Saulter, ONR) and were testimony of how versatile the instrumentation of process control can be. In addition, if there are government monies driving the development of new instrumentation, we will all benefit, in the long run. Remember the moon landings and Velcro and Tang?
As usual, Ajaz Hussain (Sandoz) had a lot to say. His enthusiasm has not diminished a whit since moving from government to industry. His quality by design campaign encompassed everyday medicines through the (potential) bird flu pandemic. However, he didnt include many specific actions in his department. But to be fair, hes only been in industry for a couple of months, so he is probably still putting his staff into place.
The discussion on ASTM E-55 was interesting, though. I was able to fill in the missing pieces when Pat Picariello (ASTM International) outlined the mission and breadth of the ASTM organization. I knew it had been around for a while, but I didnt realize it came about in 1898. There are currently over 12,000 standards (procedures, definitions, methods) under the ASTM umbrella, with 125 countries participating. Being a chemist, I appreciate the depth and breadth of the ASTMs accomplishments over the years. Industries have basic compatibilities now, from angles and spacing of threads on screws to the standard manner of testing for octane ratings.
I am sure they will bring the same thoughtful and measured approach to pharmaceutical process analysis and control. In its most obvious first step, the committee is putting out a lexicon of terms. Considering that we are still not sure of what the A in PAT actually stands for, this is a very good start. I look forward to seeing some of the other committee reports.
The only caveat I would offer is that pharmaceuticals are different from tar paper, machine screws, potato chips, motor oil and plastic bags. There are a number of legal barriers to industry-wide standards being accepted. And while a majority of companies may agree on standards, there are legal precedents for standards being set by other bodies, for instance, the USP and the FDA. If commonly agreed upon industry standards were allowed to take precedent over law, cGMP could be ignored by consensus. While it is true that the USP and the FDA have only one vote each in the ASTM committees, they are both bound by the laws Congress enact and, in reality, are 800-pound gorillas in drug manufacturing.
Some pretty nice hardware was discussed, too. (That is my favorite topic.) NIR tunable laser spectrometers were discussed by Mickey B. Frish (Physical Sciences, Andover, Mass.). While the thrust of the talk was light gases (methane and carbon dioxide) and their greenhouse effects, the hardware was impressive. I can see this being applied to off-gases from bioreactions and drying processes in pharmaceuticals.
Made with the same technology as computer chips, it is fascinating in concept and practice. Virtually explosion-proof in design, it requires no containment cases for industrial applications.
Another unique engine, derived from the telecommunications industry, was discussed by Mike Butler (Polychromix, Inc., Wilmington, Mass.). The spectrum emitted is controlled by a MEMS surface which reflects combinations of the light reflected from a sample. Using a single detector, these signals are de-convoluted into a usable spectrum. Also quite small and inexpensive, the engine lends itself to portable, hand-held devices. They can be self-contained (computer on-board) or used as a wireless device in conjunction with a home-based computer in a process setting.
Laser-Induced Breakdown Spectroscopy (LIBS) was introduced for aluminum and glass applications, but it has pharmaceutical applications as well. The small point of attack of the powerful laser causes an emission plume. This is read for elemental analysis; metals and some heavier non-metals are detected and quantified. While not addressed in this talk, I have seen LIBS used to measure titanium in the coating of tablets and magnesium (from Mg stearate) throughout the tablet. This shows the distribution of both coating materials and lubricant in a single experiment. This is good since the propensity of formulators to use TiO2-based coatings tends to preclude spectroscopic analysis of the core after coating.
Two of my favorite talks were by the same person: Fiona Clarke of Pfizer. The first was on using chemical imaging for root cause analysis (RCA). Her talk centered on understanding the process and avoiding problems, not just solving why a product failed after the fact! The concepts were to find factors that were:
- critical must be measured, as it will impact final quality of the product, or
- key is important, but probably wont affect final quality of the product.
Also, the analyst must determine if the particular attribute is a Special or Common cause. In other words, is it something to be controlled or avoided?
She used CI for this project with stunning results. She also determined which attributes were Critical to Quality Attributes (CQA) or Critical to Process Parameters (CPP). That is, she identified whether or not the differences noted were going to affect the production of the product or its final performance. While she mentioned mid-IR, near-IR, terahertz, x-ray fluorescence and Raman imaging, she stuck to NIR.
Some process examples were relating the change in hydration state of Mg stearate and variation in API particle size to dissolution parameters in the finished product. Overall, the types of information she has gleaned from her RCA work and where in the process it can help are:
- During Process Development: She studied the effect of increasing roller compaction force on tablet matrix and product performance. For example, it seems that increased force increased the number of Mg stearate domains and changed dissolution performance.
- Effect of Scale-Up: The degree of micro-mixing and lubricant distribution varies at different scales. The lab scale retains pure component domains, while scale-up shows more intermediate micro-mixing.
- Dissolution Performance: A variation in granulator performance changes the number and size of component domains. A variation in blending changes the number of component domains. Variation in input API particle size affects the number and size of component domains.
- Disintegration Issues: Variable disintegration may be related to number and size of components.
- Sticking/Capping Issues: Variation in blending causes the number and size of component domains to vary, leading to both sticking and capping.
- Variation in Components: A difference in water content as seen in the spectra can lead to flow issues. There is a variation in the distribution of components, seen in the ratio of clumps/fines and nearest-neighbor effects.
Without sounding like Fionas press agent, her other talk was interesting to me as an NIR person and physical chemist. In her second paper, she shared some wonderful work based on empirical and theoretical data. The purpose was to determine the size of a sample seen when doing NIR reflection spectroscopy. The results, which I am sure she will publish, were applicable to microscopy, imaging and macro NIR spectroscopy. I have always made assumptions as to depth of penetration and sample size; now I can make educated guesses based on data.
Perhaps the largest increase in number of applications was in the field of Raman. I was impressed that Tuesday saw an entire session devoted to the subject. Vendors (Kaiser Optical, Ann Arbor, Mich., and Horiba Jobin Yvon, Edison, N.J.), academics (University of Washington and University of British Columbia, Canada), industry (Bristol-Myers Squibb), and the FDA (St. Louis) all reported applications of Raman. Indeed, the hardware and accessories have come a long, long way since the early 1970s when I was destroying samples at Rutgers
Exhibits
Normally, I enjoy strolling through a vendors exhibit area in hopes of finding one or two new-ish items (and picking up freebies for my wife). My heavens, for a (relatively) small exhibition, it was packed with booths I wanted to visit (and not for the gifts). The good part, for serious attendees, was that there were no mega-booths as at, say, PittCon. They were pithy and to the point. All the booths were staffed with people who could answer questions, not just hand out brochures. I was quite pleased. AND there were new twists, even on established instrument lines.
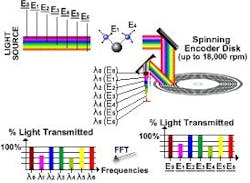
Aspectrics (Pleasanton, Calif.) introduced a nifty Encoded Photometrics Infrared Spectroscopy (EP-IR) device. This device contains a rotating encoder disc that can measure up to 256 specific channels simultaneously, moving at a rate of (up to) 100 scans per second. The disc resembles a CD, but is far more. The light is shone on a sample and the reflected light collected.
Software is very, very critical to the PAT success story. We needed something more than the glorified calculators I grew up with. The computer companies have done their jobs, and now it is up to the software companies.
I was impressed by the Symbion package that Axiom (Irvine, Calif.) has assembled. It seems to be a nice answer for how a process stream can be monitored, in real time, by a myriad of unrelated systems. Analysis and control are the themes they operate under.
Ometric (Columbia, S.C.) also seems to have a novel product. While I didnt get to spend much time with the reps, I am impressed at the bite they have taken and are attempting to chew. They are claiming to be able to control everything from raw material analysis through blending, drying, compressing and packaging with their software. I need to learn more, but, assuming they can meet their promises, this could be a company to watch.
This year, all the magazine booths were actually manned! Some were tables with magazines last year, but this year had the editorial staffs present. Of course, I have to mention the fine, fine vehicle for this article, but there were others, too.
Alphabetically, they were: American Pharmaceutical Review (Russell Publishing), Control (Putman Media), Journal of PAT (Russell), LC/GC (Advanstar Communications), Pharmaceutical Manufacturing (Putman) and Spectroscopy (Advanstar). This may not sound like much, but it is another sign of the growing awareness of IFPAC by the media.
Networking
One problem I have with the new generation of scientists is they seem to be losing the art of networking. The internet has replaced professional societies (which are all losing membership: SAS, ACS, etc.) in their minds. The value of sitting down with peers and older members of the art is often missing. But, I was thrilled to see the number of clusters of attendees, vendors and FDA personnel sitting, standing, clustering and TALKING! They werent discussing the Olympics, either. They were sharing experiences and ideas on analyzing, digesting and validating data. They were gathering knowledge on recent breakthroughs, Agency rulings and guidelines, and potential problems facing implementation of PAT.
People, I felt as though I were back in the good old days of meetings where we actually met other scientists. I had so many conversations happening at the same time, I almost forgot to attend paper presentations, which in turn were distracting me from exhibits. It was one of the busiest meetings I ever attended, and I have been going to meetings for a long time. Since the top names in pharmaceuticals and the FDA were there, I expected nothing less. Nonetheless, it felt good to see all the groups actually sharing information, opinions and plans. The networking alone was worth the trip.
The sit-down facilities were excellent, with comfortable chairs, tables and even some rooms available for interviews, business discussions or just get-acquainted chats. The ambiance was just right for trading ideas and we were far enough away from the bright lights of the city to not be lured downtown during conference hours.
I am already planning on coming back next year.