Six Sigma, the Baxter Way
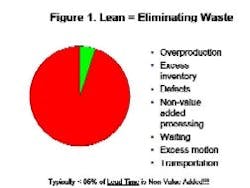
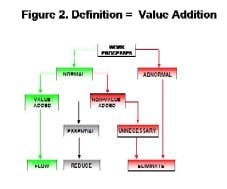
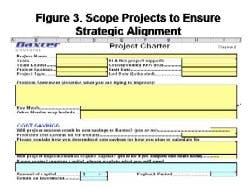
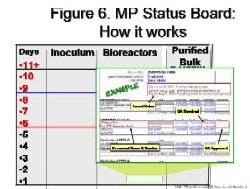
By Agnes Shanley, Editor in ChiefBaxter Healthcare is one of a handful of pharmaceutical companies that have made operational excellence part of their corporate culture. The companys North Cove plant in North Carolina won the Bronze award in Pharmaceutical Manufacturings 2005 Team of the Year Award, and the first prize in ASQs Team Excellence awards this year.The same principles that have guided that team are also achieving results at the companys other facilities. Six Sigma and Lean tools are helping operations staff improve efficiency and quality at Baxters biologicals plant in Hayward, California. At the Emerson Users Group meeting in October, Pharmaceutical Manufacturing caught up with Director of Operations Allen Harmon, to find out how his staff is using Lean and Six Sigma concepts to drive operational excellence at the facility.Pharmaceutical Manufacturing: What led Baxter to implement Operational Excellence programs at its Hayward facility?A.H.: Baxter already has a well-established manufacturing excellence program in place on the corporate level, and supports these efforts at each of its sites. A key business for us at Hayward is the contract manufacturing of biologicals, a market that has become increasingly competitive. When capacity was scarce, it was a sellers market, but theres a substantial amount of overcapacity today and cost is becoming a critical factor for sponsors, and a key determinant of whom they choose as a contract manufacturing partner.PhM: Have CMO fees dropped recently?A.H.: Some analysts predict that fees will fall by 25% or more over the next few years. And there are significant expansion plans slated by Cook Pharmacia, Cytovance, and possibly Cardinal and a company called Cobra, which plans to expand its protein manufacturing capabilities. Lower cost offshore competitors are also expected to enter the market.PhM: Has that exerted pressures on margins?A.H.: The trend is definitely being seen throughout the industry. At Baxter and Hayward, we needed a mechanism to compete more effectively in this highly competitive and volatile business environment. We needed to explore all opportunities to reduce cost and improve operating efficiencies at the site, which develops and manufactures monoclonal antibodies, therapeutic proteins and other products.PhM: How large is the Hayward facility and the companys biologics business?A.H.: The Hayward facility employs 100 people and uses 15- to 1500-L scale bioreactors and other equipment. Baxters Bioscience division accounts for roughly one-third of the corporations business and about $3 billion in sales each year.PhM: What makes Baxters approach to Operational Excellence different from that of other drug companies today?A.H.: The corporation treats each facility as if it were an independent small business. Each site must develop its own five-year strategic plan, and is responsible for profit and loss. The corporate BioScience Division also provides support, offering continuous improvement expertise. Baxter benchmarks best practices internally, and holds annual continuous improvement forums for plant leaders.PhM: What changes in any companys corporate culture are needed to establish a culture of operational excellence and continuous improvement?A.H.: Operational Excellence requires developing and rewarding the best operational performers in a manner that is customer-focused, fact-based, process-oriented and results-driven. The foundation for establishing this culture has already been established at Baxter, and efforts are applying these concepts at each facility.PhM: How did you approach establishing the operational excellence program at Hayward?A.H.: We took a top down approach, starting with strategic objectives. We then figured out what organizational structure wed need, and did some value-stream mapping to determine the gap between where we are and where we want to be. The next step was staffing cross-functional teams with representation from key areas, including manufacturing and operations, mechanics, quality, engineering. Finally, we determined which products we needed to focus on, and used elements from Six Sigma, Kaizen and Lean to analyze where improvements could be made, and which improvements would have the most significant impact on quality. These efforts involved team members as well as Green and Black Belt specialists.PhM: What principles guided your work?A.H.: Six Sigmas DMAIC methodology is critical. You need to define the problem in terms related to the customer, measure performance and know your data are good. You also need to analyze, to look for root causes, determine and confirm that you made the best choices and control the whole process so the problems wont recur.DMAIC requires a hard-data-driven approach. If you cant express what you know about any process in the form of numbers, then you dont know much about it. And if you dont know much about it, you cant control it.
- New business;
- Operational excellence;
- State-of-the-art operations;
- Excellence in quality and compliance;
- Better connection with employees.