Advanced Process Control: Bridge the Gap
Aug. 30, 2005
12 min read
A thirty-year focus on risk aversion has left a deep wound in the pharmaceutical industry. Manufacturers are still far more focused on avoiding mistakes than they are on continuously improving their processes. For many, quality is still measured more by the degree of error-free documentation than it is by fundamental process knowledge.Nowhere is this wound more apparent than in the drug industry’s slow adoption of process control and automation — in particular, advanced process control technologies. Advanced Process Control (APC) software, widely used in the petrochemical and food industries, manipulates key process variables to achieve one or many objectives simultaneously; for example quality, yield, energy efficiency and waste reduction.By providing a direct, real-time connection to any drug manufacturing process and APC platforms, Process Analytical Technology (PAT) opens up new possibilities for manufacturers.While cultural issues remain a challenge, the basic infrastructure required to sustain PAT, both for IT and for automation, has not yet become widely deployed within the industry.In fact, the degree of “evolution” or readiness for advanced control varies between drug companies, and even between different divisions or manufacturing plants within the same company. Some facilities may be highly automated, using manufacturing execution systems (MES), distributed control systems (DCS), electronic batch records (EBR), data historians, laboratory information management systems (LIMS), asset management systems, and enterprise wide resource planning (ERP) software. Others are limited to more primitive chart recorders, and operators must record data manually onto paper batch records. These differences have a staggering impact on a manufacturer’s ability to analyze data and control processes, to take effective investigational corrective and preventative actions, and to continuously improve processes.Nevertheless, the pharmaceutical industry is evolving toward more advanced process control as it embraces the concepts and technologies behind PAT. Many pharmaceutical companies are investing in sensors and analyzers (e.g. NIR, mass spectrometry, Raman) and evaluating, if not applying, them across a wide range of processes and locations from development scale for full production scale.Control what matters – the end resultA key principle guiding the PAT initiative is the vision of achieving enhanced process understanding. According to the FDA’s PAT Guidance for Industry “a process is generally considered well understood when:
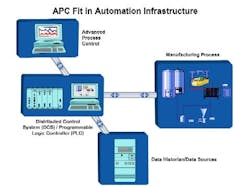
David A. Radspinner, Ph.D. joined Thermo Electron Corp. in May of 2005 as PAT Development Director and is located in Madison, Wis. He works on delivering PAT-related solutions for the pharmaceutical industry in areas of enhanced measurement systems, data management, process monitoring and control, process risk management, process mapping, and real-time release. He also helps customers define and implement their PAT strategy. Radspinner joined Thermo Electron from Sanofi-Aventis Pharmaceuticals, where he was the Drug Product PAT leader of their strategic PAT initiative. He developed a scientific, risk-based approach to PAT at Sanofi-Aventis on a full-scale production process for a high volume drug product leading to the first major comparability protocol filed and approved with the FDA.Matt Tormollen joined Pavilion in January of 2003 and has more than 20 years of software industry experience. He is responsible for Pavilion’s worldwide market development, product management, communications and branding. Tormollen has been instrumental in supporting the company’s PAT solution offering, including multivariable data analysis, Soft Sensors, and advanced process control. Figure 1: APC in Relation to the Manufacturing Automation Hierarchy
- all critical sources of variability are identified and explained;
- variability is managed by the process; and,
- product quality attributes can be accurately and reliably predicted over the design space established for materials used, process parameters, manufacturing, environmental, and other conditions.”
- Developing manufacturing processes in the laboratory and the pilot plant that meet FDA standards for quality and control which may include process modeling to further enhance the success of tech transfer to the full scale manufacturing;
- Following procedural recipes for the manufacture of drugs with limited if any adjustment of the process;
- Conducting periodic lab samples to test quality;
- Monitoring process parameters that may or may not be directly related to critical quality attributes.
- Enhanced ability to determine product quality in real-time, on-site without waiting for a lab sample or an end-of-the-run analysis.
- This capability is provided by the ability to predict quality accurately in-process and the improved process stability.
- Improved operations stability and consistency.
- The product and batch transitions are performed more quickly and consistently. This capability translates directly to improved operational efficiencies and transition losses are reduced.
- Ability to push process constraints.
- APC reduces process variability, enabling manufacturers to run closer to desired targets and push to constraints with more confidence. The result is more throughput, higher quality product or both.
- Increased operational efficiency.
- This is measured in terms of reduced raw materials and energy consumption.
David A. Radspinner, Ph.D. joined Thermo Electron Corp. in May of 2005 as PAT Development Director and is located in Madison, Wis. He works on delivering PAT-related solutions for the pharmaceutical industry in areas of enhanced measurement systems, data management, process monitoring and control, process risk management, process mapping, and real-time release. He also helps customers define and implement their PAT strategy. Radspinner joined Thermo Electron from Sanofi-Aventis Pharmaceuticals, where he was the Drug Product PAT leader of their strategic PAT initiative. He developed a scientific, risk-based approach to PAT at Sanofi-Aventis on a full-scale production process for a high volume drug product leading to the first major comparability protocol filed and approved with the FDA.Matt Tormollen joined Pavilion in January of 2003 and has more than 20 years of software industry experience. He is responsible for Pavilion’s worldwide market development, product management, communications and branding. Tormollen has been instrumental in supporting the company’s PAT solution offering, including multivariable data analysis, Soft Sensors, and advanced process control. Figure 1: APC in Relation to the Manufacturing Automation Hierarchy
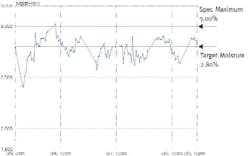
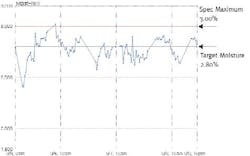
Figure 2: Fonterra Moisture Variability (before APC)
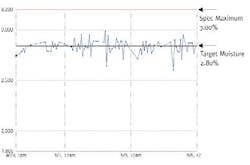
Figure 3: Fonterra Moisture Variability Reduction (with APC)
About the Author
David Radspinner
Ph.D.
Sign up for our eNewsletters
Get the latest news and updates