Making the Case for Continuous Processing
Pharmaceutical manufacturers are using continuous processing but have not been quick to convert from batch to continuous processing, due to concerns about the difficulty and risk of process change. But the basic processes that are used in pharmaceutical manufacturing, such as wet mass granulation, encapsulation, powder wetting, and addition of multiple ingredients have been run on a continuous basis for many years in the food and chemical industries.
This article will explore some of those proven methodologies for continuous processing that may be the basis for many of the existing pharmaceutical manufacturing processes.
It’s Been Done
The types of processes used to manufacture a typical product, regardless of exact formulation, may involve some or all of the following processes or operations:
- Addition of some type of fluid to a base (wetting)
- Microscopic homogenization of an ingredient throughout the base (dispersion)
- Completing the reaction of the active drug with the base to achieve the intended result (reaction)
Instead of examining the entire process, break it down to its individual operations, then ask: Have any of the operations been conducted on a continuous basis, and if so, what knowledge could be gained from that experience? In this brief summary, we attempt to do that for the hypothetical process above.
Wetting: There are many applications in operation of simple continuous wetting of powders. The liquid content of these powders can range from a few weight percent to as much as 50 weight percent. Production rates range from kg/hour to hundreds of kgs/hour. Although it is used primarily in the food industry, many chemical and specialty industries also use this process.
Dispersion: The combining capability of continuous processing extends to as high as 99 to 1 (% base to % active addition) with complete dispersion and repeatable results. This is also being accomplished at multiple levels of throughput rates and under a wide range of viscosities. The products involved cut across a wide spectrum of food and chemical applications.
Reactions: In the continuous process, it is possible to utilize the shearing intensity of continuous mixing to conduct reactions within the process and still maintain high through put rates. This is possible because of the tradeoff between time for intensity—in this case, one applies high shear to a small amount of product for a short period of time. This is the opposite of long cycle times of large batch processes, and has been done successfully in many food and chemical applications for decades, as shown in the Table 1.
| PROCESS OPERATION | TYPICAL PRODUCTS |
| Shearing/Kneading/Heating | Medical/surgical, adhesive, nicotine patch, chocolate conching, cocoa dutching |
| Shearing/Kneading/Cooling | Sorbitol, lethicin medication, Schaffer’s salt, hot breaking of vegetables |
| Reactions | Herbicides, pigments, sodium benzoate, cellulosic ethanol |
| Wet Mass Granulation | Time released blood pressure medication, hormone dispersion, coatings |
| Grinding | Peanut butter pastes and crèmes, microcrystalline cellulose (MCC), chitosan production |
| Crystallization | Vitamin E, sorbitol, by-pass fat |
| Wetting | Carbon black (pacemaker battery paste, motor brushes, magnetic ink), dairy powder (cheese mixing and cooking) |
| Ingredient Encapsulation | Confection flavoring, drug time release, PVOH, Zein solution coatings |
| Additions of Fillers | Composites, wall board, nutrition bars |
| Homogenization | Nutraceuticals |
Table 1. Process Steps Successfully Converted to Continuous Processing
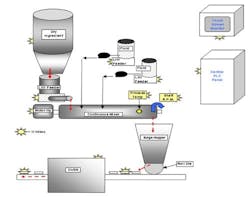
Figure 1 illustrates a process that is already being used by many food manufacturers. In this example, materials are fed via feeders, pumps or other conveying method, by electronic control of each piece of equipment and of the process itself, into a single processing unit. The design of the processing unit enables these operations to take place in a continuous method, in order to achieve the same results as in the original manufacturing process, done in separate batch type method.
Figure 1
The general principal of the continuous approach allows the use of a wide variety of equipment to provide continuous, metered feedstock of single or multiple dry ingredients, that are combined with single or multiple liquid type ingredients. The characteristics of the ingredients will dictate the size and type of feeding and pump equipment needed for the application. However, it is possible to obtain equipment that will allow a broad spectrum of ingredient types to be fed, metered, and controlled in the processing unit.
The single processing unit can be configured to provide customized mixing conditions for product specific designs, and highly controlled, consistent results.
Control System Concerns
One of the major drawbacks when evaluating continuous technology is the difficulty or inability to design both a centralized and simplified control system that will accept a multitude of input signals from disparate manufactures of support equipment. Many manufacturers of support devices such as pumps, feeders, and conveyors also provide proprietary electronic control packages for their individual pieces of equipment. Attempting to communicate with all of these “black-boxes” is a significant barrier. This attempt to specialize their product for a distinct market advantage makes system integration much more complex.
Technology now exists that allows manufacturers to overcome this electronic roadblock, while still retaining the process control reliability that is needed to insure product integrity. The basis for this is programming logic is specifically developed to control all of the required functions of the continuous process, via a PLC-based open architecture and universal controller.
There are different ways to achieve this open control, and many equipment and process control vendors offer solutions. Melfi Technologies, a systems integrator based in Toronto, uses Allen-Bradley ControlLogix hardware and Rockwell Automation RSView software platforms to achieve a PLC-based open architecture and universal controller that can accept any manufacturer’s instrument input. The blending control system integrates all discrete components onto PLC hardware. Process temperatures are monitored directly by a PLC thermocouple card. All shaft speeds are monitored directly by a PLC tach encoder card. Both the powder and liquid loss-in-weight scales are monitored directly from load cell amplifiers by the PLC over an Ethernet IP network. And finally, all valves and motors are controlled directly for the PLC I/O cards.
By integrating the blending, heating, cooling, and feeding control operations, redundant monitors can be installed to allow the blending line to be operated from multiple locations, for instance, blender, the feeder mezzanine, the operator control room, or remotely via modem or VPN connection. The integrated blending control system performs:
- Closed-loop PID temperature control of all heating and cooling zones.
- Closed-loop PID gravimetric control of all in-feed materials.
- Recipe database for selecting preprogrammed formulations.
- Live trending, recall, and data logging of all process variables.
- Multiple layers of security access to ensure process integrity.
A typical display of the touch screen controller is shown here.
Bren Kindelsperger is the President of Readco Kurimoto LLC, a manufacturer of continuous mixing machinery.
Delmar Schmidt develops PLC-based control systems for gravimetric feeding, and has worked both directly and as an outside representative for the major feeder manufacturers. He is the national sales manager for Melfi Technologies, a control systems integrator specializing in dynamic weighing systems for powder and bulk solids handling.