It is critical to operate manufacturing equipment properly to ensure product quality and process stability. Current good manufacturing practice (CGMP) requirements stipulate that the manufacturing process must be performed on qualified equipment before being validated. It is a regulatory expectation that pharma manufacturing facilities, systems, utilities and equipment are designed, constructed and qualified to suit their intended purpose.
In their guidelines, regulatory authorities indicate that organizations must build quality into their processes and products. As an example, in 1962, the FDA dedicated Subpart D of FDA 21 CFR Part 211 to equipment regulation. With the advancements in technology, the regulation was further enhanced in 1978 through 43 FR 45077 to include automatic, mechanical and electronic equipment.
The equipment the pharma industry used to use was basic. Major activities, such as adding ingredients to the manufacturing process, setting machine parameters, and transferring in-process or intermediate-stage materials and finished goods to manufacturing sites, were predominantly carried out manually.
When we think of equipment today, we do not visualize a simple piece of equipment but rather a complex system that is not only efficient, consistent, compact and compliant but also autonomous (with minimal human intervention) and connected (with IIoT, data lakes, smart analytics technologies and more). The idea of equipment sitting stationary on the shop floor has been replaced by equipment that can change manufacturing dynamics.
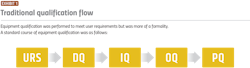
Therefore, the traditional validation method (see Exhibit 1) for complex systems can be redundant, time-consuming, costly and could pose challenges in real-time monitoring. To address these issues, the industry should be turning to cutting-edge technologies to streamline and enhance equipment validation processes.The future of equipment qualification
Historically, industry held a more cautious approach towards adopting technology, appreciating the potential of change but hesitant to fully embrace it. Equipment and instruments were designed with simplicity in mind, relying on hard-wired connections or with minimalistic computer control (basic PLC). Industry reluctance stemmed from a limited understanding of the advantages offered by the newer systems, as well as limited clarity from regulators on how to navigate the transition from manual to automatic systems while preserving data and its integrity. However, with a growing emphasis on patient safety in ISPE’s GAMP guidance and the ISPE Baseline Guide: Commissioning and Qualification guidelines, a fundamental structure is being ratified that ensures that safety is built into every stage of the equipment life cycle. This is an ideal time to innovate in the field of equipment technology.
While there is an upsurge in demand for equipment connected to the internet or within a company’s data servers (data lake), there is also an uptick in the workload added to the validation team at manufacturing sites to deliver the equipment on time for commercial operations. As equipment continues to increase in complexity, its qualification becomes more complex. It is crucial to ask some obvious questions about managing the commissioning and qualification of such systems. For instance:
- How can we reduce costs and save time during product design?
- How complicated would it be to qualify such equipment?
- How do we simulate the failure mode of the equipment or its computer system?
- How do we tap into the potential of process optimization, reducing variance and predicting maintenance?
- How do we simulate test conditions without impacting the delivery schedule?
- How do we ensure that equipment does not fail during real-time operation?
- Can the system provide auto-generated data for the tests that were pre-defined during system design while managing all the iterations that have taken place
The answers to these questions lie within one of the more recently recognized technologies of our time — the digital twin.
A digital twin is a virtual representation of a physical asset, system or process. It involves using real-time data from sensors, advanced analytics and modeling techniques to create a virtual counterpart of the physical equipment. This twin can be used to monitor, analyze, and optimize the equipment’s performance, troubleshoot issues, or simulate various scenarios without affecting the actual system.
Digital twins in equipment qualification
There are several applications of this technology for qualification and validation in the pharma industry, such as:
- Design and simulation: During the initial design phase, a digital twin can be created to simulate the equipment’s behavior and performance under various conditions. This allows engineers to optimize the design and identify potential issues before the equipment is physically built, reducing the risk of costly errors and rework.
- Virtual qualification: Digital twins can undergo a virtual qualification process where they are subjected to the same tests and criteria as the physical equipment. Comparing the performance of the digital twin to the actual equipment can help verify if the equipment meets regulatory and quality standards.
- Process validation: Digital twins can be integrated into the pharma manufacturing process to monitor and validate critical parameters and performance. By comparing real-time data from the physical equipment with the digital twins’ simulations, deviations or anomalies can be quickly identified and addressed.
- Predictive maintenance: Digital twins can facilitate predictive maintenance by continuously monitoring the performance of the equipment and predicting potential failures or maintenance needs. This proactive approach can minimize downtime, improve equipment reliability and extend its lifespan.
- Regulatory compliance: Digital twins can assist in maintaining compliance with regulatory requirements by providing a detailed and accurate representation of the equipment and its behavior. This helps in demonstrating the equipment’s reliability and adherence to regulatory standards.
- Training and optimization: Digital twins can be used for training operators and engineers, allowing them to virtually interact with the equipment and gain valuable experience without the risk of operating the actual equipment. Additionally, digital twins help to optimize processes by running simulations to identify the most efficient operating parameters. By incorporating data and information throughout the lifespan of the equipment, it is possible to exchange data with other digital twin simulators and programs.
These connections and interactions allow the digital twin to be a vital decision-making source during the equipment’s lifetime. Although this technology is gaining popularity in many sectors, it is still in its early stages of adoption in the pharma industry.
Digital twin benefits
By utilizing digital twin technology for qualification and validation, pharma manufacturers can see several benefits, including:
- Real-time monitoring: Digital twin technology allows pharma companies to continuously monitor equipment during the validation process. This real-time monitoring enables the detection of deviations and anomalies, ensuring prompt corrective actions and reducing the risk of manufacturing defects.
- Enhanced data analytics: By integrating various data streams into the digital twin model, the pharma industry can gain deeper insights into equipment behavior and performance. Data analytics help identify patterns, predict potential failures, and optimize equipment settings for improved efficiency and reliability.
- Cost and time savings: Traditional equipment validation processes involve considerable time and expenses. Digital twin technology reduces the timeline for creating physical prototypes, accelerates the validation timeline and minimizes the need for costly trial-and-error approaches.
- Risk reduction: Pharma companies must adhere to strict regulatory requirements. The use of digital twin technology can help lower risks by ensuring that equipment complies with the necessary regulations and guidelines from the outset.
- Simulating various scenarios: Digital twin enables the simulation of different operational scenarios without interrupting actual production. This capability allows manufacturers to assess how equipment responds to varying conditions, helping to optimize processes and avoid potential downtime.
- Predictive maintenance: By continuously monitoring equipment performance, digital twin technology can predict when maintenance is required, thereby reducing the chances of unexpected breakdowns and unplanned production interruptions.
A working example
A pharma company can adopt digital twin technology to validate a critical blending machine used in the production of oral solid dosage forms. The machine’s sensors can collect realtime data on variables like temperature, pressure and mixing speed. This data can be fed into the digital twin model, creating a virtual replica of the blending machine.
During the validation process, the digital twin should accurately mirror the behavior of the physical machine. Deviations from standard operating conditions would be detected promptly, allowing the company’s engineers to make the necessary adjustments in real time. The virtual model should also help identify optimal operating parameters, leading to improved blending efficiency and product quality.
Digital twin technology offers significant advantages for equipment validation in the pharma industry. By providing real-time monitoring, enhanced data analytics and the ability to simulate different scenarios, it streamlines the validation process, reduces costs and improves the overall reliability of manufacturing equipment.
As the technology continues to evolve, its adoption is expected to become more widespread, transforming how pharmaceutical companies validate their equipment and ensuring they remain at the forefront of compliance and quality standards.
Best practice digital twin approach for pharma equipment qualification
Identify equipment and requirements: Identify the pharmaceutical equipment to be qualified; list the regulatory requirements and standards that need to be met.
Data collection and model creation: Gather relevant design and operational data for the equipment; create a digital twin model using the collected data.
Calibration and validation: Calibrate the digital twin model to ensure it accurately represents the real equipment; validate the digital twin’s performance against historical data and real-world measurements.
Simulation and testing: Use the calibrated digital twin to conduct virtual simulations and tests; evaluate the equipment’s performance under different scenarios and conditions.
Deviation analysis: Analyze any discrepancies between the digital twin’s predictions and real-world data; identify potential causes of deviations and areas for improvement.
Optimization and adjustment: Make necessary adjustments to the digital twin model to improve accuracy and alignment with the real equipment; optimize the digital twin’s parameters for better predictions.
Verification and approval: Verify the updated digital twin model against real-world test data; obtain approval from relevant stakeholders for the qualified digital twin.
Ongoing monitoring and maintenance: Implement a system for ongoing monitoring of the digital twin and real equipment; perform regular maintenance and updates to keep the digital twin accurate and up to date.
Equipment qualification report: Generate a comprehensive equipment qualification report that includes the digital twin’s capabilities and performance validation.
Integration with operations: Integrate the qualified digital twin into the pharma manufacturing process and quality assurance procedures.
Continuous improvement: Continuously gather new data and feedback to improve the digital twin’s accuracy and predictive capabilities; use insights from the digital twin to drive continuous improvement in equipment and processes
About the Author
Saurabh Joshi
Director, Solutions Engineering, ValGenesis Inc.
Saurabh is on a mission to help life science companies build a robust digital roadmap. For over two decades, he led quality in operations, qualifications-validations, QMS, centres of excellence, and consulting. Saurabh has a Bachelor of Pharmacy degree and has worked with many renowned pharmaceutical companies, such as Cipla, Micro lab, Wockhardt India/EU, and Sun Pharma, to name a few. He has partaken in many regulatory inspections of USFDA, MHRA, HPRA/IMB, and WHO. As a domain expert, Saurabh advises life science companies on appropriate digital solutions to help resolve their underlying problems. He firmly believes that going digital will help organizations become more resilient and sustainable.