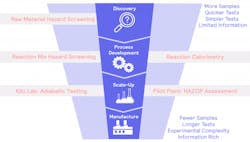
One of the biggest challenges faced in bringing new drugs to market is scale-up: taking small, lab-based syntheses through a pilot plant, to full-scale manufacture. Implementing robust process safety across three broad areas is an important step to reducing manufacturing risks. The correct approach will:
• screen for potential hazards
• evaluate main reactions, including possible unintended side reactions
• recognize the impact of “what if” scenarios to implement appropriate mitigation and control strategies
Hazards need to be identified, assessed, and mitigated at each stage of product development. Designing safety into all development processes minimizes the risks involved and delivers confidence for a safe and efficient scale-up to manufacture. It is crucial to consider when, how, and why testing is carried out.
Taking a systematic approach, the product development journey can be broken into three clear stages prior to manufacture: discovery; process development and scale-up. Testing needs to be integrated throughout the workflow to ensure safe and productive preparation, but it should be a particularly significant feature during discovery. Leaving too much lab-based testing to the process development phase is too late.
The benefits from early discovery testing
Discovery testing should be broad and typically happens at pace. It allows manufacturers to gain sample understanding at a small, cost-effective scale so that it identifies significant risks and prevents delays during process development. The data footing achieved here should shape all future decisions on how desired reactions and synthetic outcomes will be further tested.
Effective discovery testing should extend to raw materials, processes, prototypes, and end products. This top-level screening across a range of metrics will see many candidate molecules and reaction processes taken from a large group of samples, and should result in effective and rapid decision-making.
During discovery, the raw materials that are used to synthesize all desired molecules are screened for potential hazards. With Scale-up always in mind, the desired main reaction is assessed and characterized, and any potential additional thermal and pressure hazards that may occur in operation are identified. This early stage is the preferred time to evaluate potentially unsafe materials or synthetic routes, and inform the selection of alternative, lower risk options.
Micro-scale calorimetry can quickly screen the thermal properties of raw materials, but it does not provide critical information on the rate of pressure change that’s vital for assessing possible explosion hazards. It can also be a challenge to obtain representative samples of raw, pure materials and reaction mixes. This can negatively impact the rapid large sample size screening that occurs during Process Development. Preferred solutions should allow fast temperature and pressure screening on the same platform to speed up the time to manufacture. For example, a device such as the TSu Thermal screening unit enables rapid, simultaneous screening of both the temperature and pressure characteristics of a sample. It can be considered a complementary, or even alternative, technique to the classical DSC/DTA (Differential Scanning Calorimetry/Differential Thermal Analysis) methods. In comparison to methods such as DSC/DTA, the data from the TSu includes pressure change in the sample, which has the potential to be more hazardous than thermal changes.
Process safety expertise from experienced professionals working in early discovery should also feature in the decision-making process. Their insights in risk mitigation can provide great support from a manufacturing perspective, and can help to constructively shape material testing. Their demands of further testing and retesting aren’t intended to slow down discovery, but, rather to improve process development and scale-up. Reagents are used in very small amounts, so retesting is very common practice. Safety is paramount. Completing such high levels of testing in early discovery may create time and scheduling conflicts but it is required to keep simultaneous projects moving at speed.
As speed is an increasing pressure to those working in pharmaceutical discovery, effective communication has to be a priority. This is not the time to work in isolation. It is essential that everyone involved in the process, especially those with safety expertise, understands results and outcomes as they happen. Any safety-related data should be extensively shared.
Making safety an integral component of process development
From discovery comes the need for a greater understanding and the ability to define safe reaction conditions to minimize hazards and mitigate scale-up risks. Process development helps to determine a more comprehensive understanding of the desired synthetic route.
The process development phase must deliver a comprehensive approach to hazard evaluation and importantly mitigation, taken against these consistent scenarios:
• A full exploration of the desired reaction
• Avoidance of reaction thermal runaway
• Modifying operating conditions to reduce identified risks
Hazard screening once again has a role to play, alongside reaction calorimetry. Testing must identify and measure the hazards involved in selected synthesis reactions, and determine the necessary cooling capacity of the manufacturing plant to maintain safe operational conditions.
Using a variety of tools to identify, assess and then mitigate the potential hazards at each stage of Process Development, ensures confidence that a successful and efficient route to market can be delivered. Adiabatic testing and HAZOP assessments come to the fore here.
Creating safe scale-up functionality
For safe, successful scale-up, lab-based process evaluations must consider hazards at a plant level and adopt tools to mitigate their impact in worst-case scenarios. In a base illustration of scale-up challenges, large-scale reactors, like those found in manufacturing and pilot plants, typically behave adiabatically, losing little heat to their surroundings. This is very different to smaller, lab-based, vessels that proportionally lose far more heat.
This difference in heat retention is a major potential hazard for scale-up. If this was left unaddressed, the system would retain too much heat, which at best would result in more plant cooling required for temperature control, and at worst, trigger a thermal runaway reaction. The ability to fully simulate thermal runaway risks under manufacturing conditions in the lab is invaluable to ensuring that appropriate safety measures are planned and implemented prior to manufacture. If the manufacturing facilities cannot cope with worst case scenarios, processes must be modified so that the existing system can at least contain the worst case scenario.
The breadth of scale-up testing should always extend to simulating different reaction scenarios and then ensuring that the results are always robustly compared. The most effective tools for testing should be able to illustrate a variety of outcomes: from the worst-case scenario, to those with far less impact. For instance, an adiabatic calorimeter with a low Phi factor, a correction factor based on the ratio of a vessel’s total heat capacity and that of its contents, uses low thermal mass test cells and therefore responds very similarly to how a large-scale manufacturing plant would: with very little of the heat produced during reaction and runaway spent warming up the test cell. Instruments that use test cells with a high Phi factor will require the test data to be manipulated to compensate for heat losses. And, as such, a low Phi factor system will most closely mimic the behavior of process-scale vessels, at a lab-scale. For example, such as the Phi-Tec II, which allows the use of low thermal mass test cells, captures pressure change data along with information on thermal behavior of the sample. The data generated from such a system provide a basis for calculating critical safety systems, such as vent sizing.
Maintaining safety when ‘farming out’ pharma
The necessity to work with third party providers can present itself at any point in product development. When production capacity requires the use of an external operation, it is essential that consistent safety and management expectations can be relied upon. Process Safety standards have to be upheld whether the third party is working in a lab across the street, or across the globe. This is where effective management of people, processes, and resources comes into play.
Extending operations to include external players needs to be managed in such a way to feel like a collaboration with likeminded specialists. This is best achieved when outsourced service providers are seen as partners, who are very much part of the team. Under these conditions, working expectations, operations, and findings can be freely shared by and to all involved. In any example of good team management, any knowledge or skills gaps should be assessed, and key areas are identified where upskilling team members or sharing expertise will benefit the outcomes of the project. Aligning understanding and practices between all parties involved will have a positive impact on speed efficiencies and should be considered a key investment for safety maintenance.
Partnerships should also formed in a way to ensure two-way data sharing between any core and outsourced teams. The decision to work with a third party is so often made to extend testing capability, and so access to data should be distributed widely and in a timely way. Data should also be stored externally to the project to help maintain process safety at all levels; lab, plant, staff, local community, and environment.
Process safety in times of change
Even once strong partnerships are established, they can still be subject to change, and so this shouldn’t be a time for complacency. Sample retesting is essential when there are any alterations to supply, product or plant. For maintained safety, no assumptions should be made about material consistency across supplies, third party or otherwise. Small-scale sampling will be vital here to retest parameters and provide a robust understanding of materials and their associated hazards. Labs may want to do selective testing to include only the least stable reagents, but the rule should always be to test more, rather than leave anything to chance.
For a comprehensive and sustained approach to process safety, one should also bear in mind that processing sites operate differently to lab-scale environments, and this can have an impact on risks and test outcomes. The best standpoint to take to minimize delays and leave safety uncompromised is to always seek out opportunities for process improvement and expect process change. As the operation of different sites will vary, the associated risks can also change. Therefore, the best practice to meet safety needs is to carry out risk assessments whenever there is a location or scale change.
Process safety is essential to getting any pharmaceutical to market safely. Finding a successful route through scale-up to full pharma production requires rigorous safety protocols to be identified, adopted and maintained. The key to this is starting safety analysis early in the discovery phase and reapplying it throughout the development process. Involving collaborating networks that are a true extension of existing operations and developing these relationships should provide fully productive discovery, process chemistry and commercial routes to manufacture. Process safety should always be applied, developed, and reapplied.