Brand Protection: Not a Cost, But a Value Enhancement
June 15, 2005
6 min read
Counterfeiting in the pharmaceutical industry is a prevalent factor in the mainstream media, with news stories and television reports hitting the public almost once a week. And the number of pharmaceutical conferences devoted to product security is at an all-time high. Yet, there is a widespread perception that not much is being done to protect the drug supply chain and help ensure consumer safety.While pharmaceutical companies are focused on spending significant marketing dollars in creating their brands in a shrinking new drug supply market, not enough attention is being given to protect these same brands. This article examines the trends in brand protection and the driving forces behind them, which will impact the adoption of security measures in a significant way industry wide.A Very Real ProblemThe United States pharmaceutical market was valued at over $270 billion in 2004 (according to Espicom), and accounts for 41.2% of the world market. This lucrative market has grown by double digits since 1995 with literally no end in sight. The attending tremendous growth and increased cost of healthcare have made this market an attractive target for counterfeiting and diversion with reports of counterfeit drugs being as high as 10% of global consumption. Yet, again, even though the problem is very real, little is being done to protect these drugs through anti-counterfeiting or other supply chain measures. One cant help but wonder why.Brand protection solutions are still viewed as a cost of goods sold (COGS) rather than a value by which they provide both the brand and the brand owner a significant image advantage and thereby increased market share. This COGS thought process forces the decisions to adopt anti-counterfeiting measures or to secure supply chains only when a particular brand is directly affected.However, significant opportunity to boost profits and enhance brands is potentially lost in this reactive approach, while counterfeiters capitalize on the profitability of the brand generated through the marketing efforts of the brand owner. This allows the counterfeiter to also emerge as a formidable competitor to the brand owner, only because the brand owner did not take the necessary security measures to protect their own brand. Let us not forget that the consumer continues to pay for all of this in the form of high drug prices.Basically, the driving forces that affect brand authentication today fall into two main areas: established trends and relatively recent trends. Together, these trends present an overall challenge to the pharmaceutical industry that can no longer be denied, or ignored.Established Trends:
- Financial Loss
These losses have always presented an obvious argument for brand owners for the implementation of brand protection solutions as they are easy to quantify as a return on investment. However, market pressure has evidently not been high enough for brand owners to take on more proactive roles in implementing these solutions. It seems that they are either unable or unwilling to quantify specific product losses to counterfeiting. - Brand Damage
Damage to the image of brand often occurs through product related crime, with sub-standard or dangerous counterfeits and/or products that have been subject to tampering. Sometimes, these events have catastrophic results, the worst of which can be consumer deaths.
Specific examples, such as the Tylenol malicious tampering incident, and counterfeit Lipitor and Viagra, have tended to spur action on the actual brands involved. But as serious as they might be, these cases have generally not stirred industry wide pro-activity in brand protection. This is in spite of a shrinking drug pipeline! - Organized Crime
Many product related crimes have become easier than ever to perpetrate, due to the widespread availability of computers, software, scanners and table-top color printers. These allow for easy duplication, especially with the increased sophistication and low cost of these technologies. It appears to be an increasingly common practice for organized crime members to utilize the manufacture and distribution of counterfeit branded drug products as a means to launder money that has been obtained illegally.
The combined low risk of detection, prosecution and imprisonment, as compared with other crimes drives criminals to counterfeiting and product piracy as a preferred activity in various parts of the world. This growth of product related crime should put more pressure on governments and companies alike to act to combat such activities. This is especially true for control drugs such as in Oxycontin. The U.S. congress has recently proposed legislation to combat all counterfeiting with stiffer penalties. However, Chinese regulations determine the penalty by the cost of counterfeit goods and not the price of goods it replaces, leading in most cases to mild penalties for the counterfeiter. In many cases in China, even the equipment is not seized, allowing the counterfeiter to get back into business quickly after paying a mild fine.
- Terrorism
The true importance and scale of product related crime to international terror organizations has only really come into the spotlight and been fully appreciated since the events of 9/11. Homeland security views terrorism as a significant threat to the nations drug supply network. This one issue, as frightening as it may be, offers an overriding reason why governments and industry, worldwide, must be more focused on brand protection and security. - Manufacturer Responsibility
In light of all the driving forces in favor of brand authentication, especially in such liability-driven markets as the U.S., it is evident that pharmaceutical manufacturers should foresee how counterfeits could enter the supply chain and/or that their product/brand could be vulnerable to tampering.
If a brand owner is considered to have failed to use reasonable efforts to secure the packaging and distribution chain from misbranded, or imitation versions of their products, then the company may be judged to have been negligent. With more and more manufacturers responsibility cases being brought before the courts by consumers, certain legal precedents will be set to force responsibility.
Cases like these are likely to encourage brand owners into making more proactive security decisions, particularly in areas where there are health and safety issues.
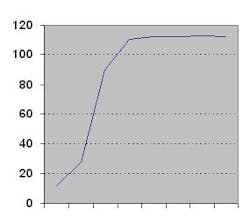
Brand protection: An emerging issueThe typical S curve at right demonstrates a cycle of adoption for any new product or solution. The trend toward brand protection in pharmaceuticals appears to be in the early adoption phase, with many brand owners looking for direction from the FDA (as related to RFID and other solutions), and from market or business models to establish value.Clearly, the trend is picking up momentum as more brands are affected. The benefits of adopting brand protection are becoming more visible; that is, the need is being strongly perceived by several pharmaceutical companies as a reaction to potential liabilities. This trend will continue for the next several years, with brand protection becoming a standard for doing business in the pharmaceutical marketplace.About the AuthorDr. Narendra Srivatsa holds a Ph.D. in Chemical Engineering from SUNY at Buffalo and a Bachelor of Technology degree in Chemical Engineering from I.I.T. Madras, India. He has been awarded numerous process and product patents, many of which have been commercialized or licensed. A member of the American Chemical Society, NJPHAST, and other professional organizations, Narendra is an innovator and a business builder with a track record of more than 16 years in industry.
About the Author
Narendra R. Srivatsa
Ph.D.
Sign up for our eNewsletters
Get the latest news and updates