For pharmaceutical companies, time really is money: those who get to market first with a new product will often capture the largest customer share and maximize profits. Drug innovators commit considerable resources to developing and seeking approval for breakthrough products, because the sooner they can market a new product, the sooner they can begin to see returns on their investment.
Time is of the essence for manufacturers of generic drugs, too, since they often have a suite of drug applications pending and they vie with competitors to be the first to commercialize their products for the 180-day period of marketing exclusivity. For these reasons, technologies and services that streamline drug development can provide important competitive advantages to drug manufacturers.
Sophisticated packaging simulation modeling can help formulation chemists and packaging engineers identify the right conditions for ensuring the chemical and physical stability of drugs. The simulations are referred to as ‘pseudo-empirical’ modeling and can be performed early in the development process, guiding production decisions and helping to avoid costly errors that could prove to be roadblocks to production.
Pseudo-empirical modeling is a technique that uses empirically derived data from the packaging materials, including moisture vapor transmission rate (MVTR) through the bottle, surface area of the bottle, sorbent adsorption isotherms and drug product adsorption/desorption isotherms. Linking these variables together mathematically will pseudo-empirically predict the relative humidity of a pharmaceutical package’s headspace and drug product hydration level over time.
This resulting information will ultimately determine the means by which manufacturers can maintain a drug’s chemical and physical characteristics throughout its shelf life.
Customized Moisture Management
Pharmaceuticals can be subject to chemical and physical degradation through interaction with moisture. This is because free moisture increases the molecular mobility within drug product formulations that can directly affect the rate of all chemical degradation pathways.
Desiccants are used to reduce free moisture within the drug product and thereby curb molecular mobility while reducing the potential for further hydration from moisture ingress through its primary packaging. There are a wide range of desiccants available to maintain pharmaceutical integrity, but it is important to choose the right desiccant type, amount and format for each drug product. Determining the best combination requires a precise analysis that takes into account the drug characteristics and packaging materials as well as the sorbent’s functionality.
Pseudo-empirical modeling can be especially helpful because engineers first define the adsorption properties of a particular drug product and then simulate the effect of that formulation in combination with a given packaging presentation with varying amounts and types of desiccants. This approach takes into account conditions during all stages of drug processing, from formulation to the packaging environment and throughout distribution.
After running this analysis, engineers can provide specifications for the best product/packaging combinations. Simulations can be performed for many types of pharmaceutical packaging in accordance with the guidelines for stability testing set by the International Conference on Harmonization for accelerated test conditions (six months at 40°C and 75% relative humidity (RH)) or real-time (room temperature) test conditions (two years, typically at 25°C and 60% RH).
Simulations at either condition require specific input criteria (e.g., isotherms, MVTR, etc.) to account for the effect that temperature has on polymers and drug products. Because of the ability to perform testing under accelerated conditions, simulations can help drug makers find a stability solution quickly, reducing sorbent ranging studies and testing time by at least six months, thus speeding regulatory filings, subsequent approvals, product launches and ultimately cash flow. It can also result in cost savings, as it enables manufacturers to purchase the precise amount of sorbent they will need for a given drug product’s packaging requirements.
Finding the Right Combination
Today’s sorbents are often described as “active packaging components” because they respond to changes in the headspace of packaging relative to outside conditions. The goals of stability testing for drug packages are: 1) to determine what the internal conditions of a drug package will be under given conditions, and 2) to predict equilibrium relative humidity (ERH) and drug product hydration over the course of the product’s shelf life.
The calculations take into account the humidity levels both inside and outside the package, as well as the drug’s and desiccant’s respective affinities for moisture at varying humidity and/or temperature levels and the rate of transfer of moisture and vapor through the package wall.
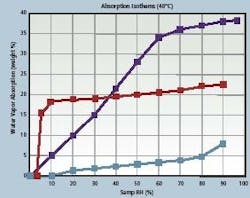
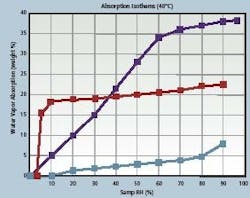
In essence, the modeling approach simulates how the interdependent variables — drug, desiccant and package — will affect one another over time. With this information, it is possible for drug makers to ensure the stability of their formulations and set appropriate expectations for their shelf lives. For example, a simulation of a solid drug packaged in a high-density polyethylene bottle and subject to moisture degradation would take into account three interdependent parameters: the moisture vapor transmission rate through the bottle wall; the adsorption isotherm for the drug (Figure 1), and finally, the adsorption isotherm for the desiccant (also in Figure 1).
Figure 1 shows the adsorption isotherms for a drug product (light blue line), a molecular sieve desiccant (red line) and a silica gel desiccant (dark blue line).
The analysis would also have to calculate the surface area and thickness of the bottle to determine possible moisture ingression through its walls. Given its performance advantages in reducing total moisture ingress, the use of a foil-laminate heat-induction seal is always assumed.
The combination of drug product and desiccant functions as a system and will reach equilibrium relative humidity (ERH) over time. When you first drop a desiccant into a bottle, it acts on all available sources of moisture, pulling moisture out of both the air and the drug. The impact on the drug product is to reduce molecular mobility as well as chemical degradation.
In this manner, the desiccant will reduce the ERH inside the headspace of the bottle, and consequently the ERH and moisture content of the drug product. For this reason, the simulation model has to calculate the required amount of desiccant to process moisture out of the drug product in order to establish a protective “envelope” of relative humidity inside the bottle.
It also has to compensate for the increased ingress of moisture that results from the use of a desiccant itself. Consider a scenario in which drug filling takes place in a test chamber at 40°C and 75% RH, and the bottle headspace conditions are at 40°C and 10% RH. The differing humidity levels will create a relative humidity gradient. Because the inside of the bottle wants to reach a point of equilibrium with the outside environment, it will essentially create a driving force for moisture to come into the bottle until said equilibrium is achieved.
Some drugs have some hygroscopic value; that is, they tend to absorb moisture from the humidity in the air. Such drugs could contribute to added moisture ingress as well, though to a lesser degree than a sorbent. An extremely low ERH and related state of disequilibrium can be observed when molecular sieve desiccants are used. In the above example, permeation takes place relative to the adsorption properties of both the drug product and desiccant. As the products adsorb moisture and the RH inside the bottle rises and begins to approach 75%, the rate of permeation slows down.
Carefully tracking this rate enables one to gauge the exact capacity that will be required from a desiccant. Additionally, the modeling technique can indicate the conditions of a specific drug product formulation if packaged without a desiccant.
Multiple Applications
Pseudo-empirical modeling can be used for a range of drug formulations, including oral solid doses, oral suspensions, powdered drugs, parenteral solutions, and active and passive transdermal technologies. Appropriate moisture management can extend the shelf life of existing drugs and make possible innovative delivery methods or drug formulations.
Consider a respiratory drug delivery device used with powdered drugs. By regulating the moisture levels inside such devices, sorbents facilitate the smooth flow of drug particles and ensure that the device works effectively and accurately each time it is used. Modeling the precise amount and type of sorbent needed for such a system to best manage moisture can streamline the design and production processes, thus reducing time to market.
Careful moisture management in respiratory drug delivery systems is critical to balancing the opposing challenges of triboelectrification [1] and particle agglomeration and resulting dosing problems. Many of today’s most innovative drug formulations are also unstable in that they are highly sensitive to both moisture and oxygen. In some cases, customized sorbent and packaging solutions developed through pseudoempirical modeling can make drug formulations viable that may once have been deemed too difficult to market.
Pseudo-empirical modeling and subsequent proofof- concept stability testing offer valuable tools to formulation chemists and packaging engineers. The information resulting from such testing enables drug makers to customize moisture management strategies to meet product needs. Stability tests also provide important evidence to regulators that pharmaceutical products will remain safe and effective throughout the supply chain.
Innovative modeling techniques bring precision and predictability to the process of choosing a desiccant and packaging solution. Registration stability testing can now be done on an accelerated time scale, speeding time to market. This, in turn, can help drug makers enhance product stability and ensure brand integrity.
About the Author
Adrian Possumato is the Global Manager - Pharmaceutical Market with Multisorb Technologies, Inc. (Buffalo, N.Y.). He has over 15 years of experience in the pharmaceutical and chemical industries. He can be reached at [email protected], tel. 908-849-3005.
About the Author
Adrian Possumato
President, Sanner of America
Adrian is a successful business development, market development and operating executive with over 30 years of experience in the pharma and health care industries and healthcare active packaging space. Before joining Sanner, he served as a principal consultant with Medica Industrial Consulting LLC. and prior to that was Vice President of Multisorb Technologies, where he worked for 20 years. Adrian Possumato holds a B.A. in Biology from Stockton University and has extensive knowledge of drug and in-vitro diagnostic product development.