If a drug product is contaminated in any way by a chemical derived from its packaging, this can cause real problems for patients. These “leachables” have caused real-world problems ranging from mild and temporary gastrointestinal symptoms to full-blown immunogenic reactions.
At the less severe end of the symptom scale, in 2009 and 2010 various products reached patients that had a musty smell. This subsequently was identified as 2, 4, 6-tribromoanisole, and eventually traced back to a breakdown product of a fungicide, with which the wooden pallets used in storage and distribution were treated.
More dangerous effects occurred in patients taking Eprex (erythropoietin alpha) after a formulation change. The solubilizing agent, human serum albumin, was replaced with the chemically derived alternative, polyethylene glycol. This change increased the propensity for the new formulation to leach cross-linking agents from uncoated syringe elastomer into the product, which then reacted with the therapeutic protein to cause an antigenic effect.
While such problems are unusual, they do occur, and thus it is essential that all potential interactions between any individual component of a drug’s formulation and any part of its packaging are evaluated carefully. Only then can the manufacturer be assured that the packaging will have no deleterious impact on the product’s safety and effectiveness.
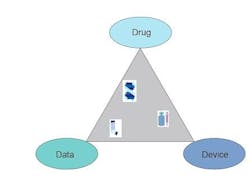
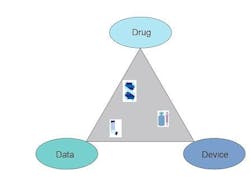
During the development and registration of every drug product, three crucial variables, drug, device and data, must be considered and understood. By considering each of these variables as the vertices of a “formulation triangle”, every study can be classified as lying within such a figure. For example, early phase studies of the action of an API in biological systems lie closest to the side connecting the Drug and Data vertices. Device compatibility studies, on the other hand, lie closest to the side connecting the Drug and Device vertices. Device performance testing can be considered to lie between the Device and Data vertices. The ultimate goal for both the developer and regulatory authority is to sample the entire space within the triangle. Only when information is available about all the possible interactions can a packaged drug product be declared safe and effective.
Extractables are those substances that can be extracted from the packaging material in some way, usually requiring the presence of strong solvents, elevated temperatures, or both. Leachables are, essentially, a subset of extractables, and require no unnatural extraction process to enter a drug product, as they are a natural interaction phenomenon between a formulation and its packaging. While both extractables and leachables might be additives that are deliberately incorporated into the packaging material, this is not necessarily the case as they might be low molecular weight fragments of the polymer, such as cyclic oligomers or even unreacted monomers.
The reactions used to make condensation polymers such as nylon are, essentially, reversible, and therefore a condensation polymer backbone can be a source of an extractable monomer created by the back reaction. Monomeric extractables are still reactive, and their free concentrations are usually low. Cyclic oligomers, such as the dimers and trimers of polybutylene terephthalate, are commonly observed extractables from this particular polyester. As long as formulation or any extraction solvent is not in contact with these materials, no observation of complete extraction will ever occur. This does not mean that such thermoplastics should be avoided as the mere presence of these chemicals may not cause deleterious effects on product quality. In fact it does illustrate that leachables will always be present in drug products and anything packaged in plastic.
The extraction process generally occurs at a solid–liquid interface, although it can occur at a solid–gas interface, particularly if a volatile organic compound is present in the packaging material. The rate of extraction depends on a number of physical and chemical factors, including the permeability of the solvent into the solid, the solubility of an extractable into that solvent, and the temperature and pressure of the system. In the laboratory, there are numerous ways in which to perform extractions. Typical techniques include Soxhlet extraction (diffusion-controlled); boiling point reflux (temperature controlled); equipment that allows elevated pressures above solvent boiling points such as accelerated solvent extraction (temperature controlled); and microwave extraction, to heat a polarisable or dipolar solvent above boiling points (temperature controlled).
The identity of extractables and leachables
Extractables and leachables fall into various chemical groups. Polymer additives are a major class of extractables, and differ both within types of polymers and even grades of the same polymer. They are added to polymers to impart desirable processing and end-use properties such as stability, and while they are not by default bad, there is the potential for them to be extracted or leach into a drug product. They cover a whole range of different functionalities, altering the mechanical, chemical or even electrical properties of the polymer.
Mechanical property modifying additives include nucleating agents that allow polymers to be processed at lower temperatures, cross-linkers that give a polymer mechanical strength and introduce a 3D structure, and fillers that impart both strength and heft. Fillers are typically inorganic substances such as clay, heavy metal oxides or carbon black. Plasticizers, which impart flexibility and give resistance to cracking, are a major class of additives, and may include both natural and synthetic oils, and phthalates. The use of phthalates is now under particular scrutiny.
Cross-linkers can also be classed as chemical property modifying additives, as they create supramolecular structures from the polymer. These larger molecules do not react or dissolve easily in any solvent, imparting chemical resistance. Antioxidants are another common form of chemical additive, and are included if the polymer has a high oxidation potential, typically because it contains many hydrogen atoms. Hindered phenols, or mixtures of these phenols with phosphine compounds, are examples of this class of additives. Flame retardants and clarifiers can also be incorporated into a polymer to make it less susceptible to high temperature oxidation.
Electrical property modifying additives are typically surface modifiers to control static build up. These increase the rate of charge dissipation at surfaces by incorporating a polar group or fixed charge within the additive’s molecular structure. Examples include aliphatic amines, amides, quaternary amines or polyol compounds.
Usually, leachables are considered to arise from packaging directly in contact with formulation; i.e. “primary” packaging. Additionally, extractables in “secondary” packaging; i.e. materials in contact with the primary packaging, and even from “tertiary” packaging, may find their way into a drug product prior to the end of its shelf life. These may be present in any part of the entire packaging and delivery system. Examples include components of printing inks, and chemicals such as flame retardants or antifungal agents that might be present in cardboard boxes or shipping containers.
Inorganic compounds can also be found as components of packaging materials. Many of these chemicals will fall into a specific additive class such as filler or, in the case of metal salts of medium chain fatty acids, plasticizers. While rare, it is possible that residual heavy metal catalysts may be detectable in plastics. Fortunately, analytical technology for the detection and quantitation of heavy metals in drug products is fairly well understood, and both the European Pharmacopoeia and the United States Pharmacopeia have well described methods and limits for these elemental impurities.
The origins of extractables and leachables
An extractable or leachable may be introduced at any point along the packaging supply chain. While a company involved in packaging pharmaceutical products will require full disclosure from their suppliers about their processes and any extractable that might have been introduced in their facility, this disclosure requirement does not extend all the way back through the many links of the supply chain.
A pharmaceutical company will source its packaging containers and device components from a moulding shop which converts polymer products, and may add, for example, lubricants and colorants during moulding. The converter sources its materials from a masterbatcher, whose processes could introduce stabilisers, antioxidants, processing aids or antistatic agents. The masterbatcher is supplied by a polymer manufacturer, whose products might contain catalysts, stabilisers, antioxidants or processing aids. The raw materials for polymer manufacture are provided by a company that synthesises monomers, where bulk chemicals or storage stabilizers might enter the supply chain.
Quality agreements between pharmaceutical manufacturers and their suppliers generally do not cover disclosure beyond the first level supplier. In the US, pharmaceutical manufacturers also have the advantage with first level suppliers because those first level suppliers generally will avail themselves of the Drug Master File (DMF) system. Having a DMF has become a marketing tool for first level suppliers and helps them obtain business from the pharmaceutical industry. The second level and further level suppliers are so far removed from the pharmaceutical industry and do not find the size of the market to be advantageous to their bottom line.
Whatever the form of the packaging, there is always the potential for leachables. Even for typically clean primary packaging, as used for small volume injectables, in cases of long-term storage, inorganics from glass or organic compounds from the elastomeric stopper may be found. If it is packaged in a single-dose pre-filled syringe, there are more potential source components, including the thermoplastic barrel, elastomeric plunger, metal needle, elastomeric needle sleeve, or even the foil pouch or plastic blister that constitutes the secondary container.
Container Closure System Components
Primary packaging components, which may be in direct contact with the drug product, and thus may contribute extractables or leachables. These include:
•Containers (ampoules, vials, bottles)
•Container liners
•Closures (screw caps, stoppers, metering valves)
•Closure liners
•Stopper overseals
•Container inner seals
•Administration ports
Secondary packaging components, which will not be in direct contact with the drug product, but may still contribute leachables under certain conditions. These include:
•Container labels
•Administration accessories
•Shipping containers
The situation is even more complex for products designed to be delivered by infusion. There is potential for long-term storage exposure from the laminate or multilaminates that make up the bag, as well as inks, thermoplastic ports and thermoelastomeric connectors. Further potential sources of may come from in-use exposure during infusion, particularly from thermoplastic connectors or elastomeric tubing.
Probably the most complex interaction and, historically, the system of most concern between primary packaging and formulation is the pressurized metered dose inhaler (pMDI). A pMDI has many components that could, conceivably, contribute to the extractables load. These include the metal canister and spring, the valve body, stem and metering chamber, the gaskets on the stem and the valve, and the gathering ring. All of these components will be continuously bathed in an organic solvent (propellant and carrier) for as long as the product is viable.
The leaching of extractables into drug product formulations is inherently a kinetic problem. Intimate mixture between formulation and the packaging will increase the rate of leachables formation. The more similar a formulation is to its primary packaging, the more care should be taken with the choice of primary packaging, and in the design of studies to show its suitability. While there have been recent examples of deleterious leachables in solid oral drug products, the rate of transport of small molecules from packaging onto such dosage forms is generally low. However, it has happened, and should be of concern if there is the potential for volatile chemicals to arise from the primary packaging, or be transported through the primary packaging. Both primary and secondary packaging materials should be screened for volatile organic compounds as part of a systematic characterization of all packaging components.
Both small molecule drugs and biologics have the potential to be contaminated by extractables, and the direct harm a leachable may cause a patient is the same in either case. There may be some dependence on site of action, which is still related to the inherent safety profile of the contaminant. However, even at “high” concentrations in a small molecule drug preparation, the amount of leachable will usually be small compared to the number of molecules of the drug, and any reactions that might take place between the leachable and the active will not have any appreciable effect on potency. In contrast, the number of molecules of large biologic drugs in a dose is much lower, so any reactions between the leachable and the biologic are much more likely to impact potency. Another issue with biologics is immunogenicity, which is very difficult to predict from chemical structure alone.
Different dosage forms of the same molecular entity will need to be studied separately. It is not possible to state, without carrying out proper investigations, that which is safe for an inhalable formulation, for example, will automatically be safe for an injected drug.
While the identity of extractables or leachables can be unexpected, some cause well known problems, and thus are either best avoided or very carefully tested for. For example, there have been concerns about the migration of benzophenone from labels into ophthalmic products. Benzophenone is a common component of UV-active inks, but is also a potent irritant. Most label suppliers are aware of this link, and offer benzophenone-free options. However, while this individual chemical may have been removed, the irritation potential of the alternative chemicals that are being used instead has not yet been established.
Another well-known problem arises from vial closures. In general, most suppliers have switched their elastomeric closures from natural sulfur-cured latex to other, synthetic, materials. The older materials can contain extremely undesirable chemicals such as nitrosamines and polynuclear aromatic hydrocarbons, which are suspected (or known) carcinogens. It is therefore advisable to use modern synthetic elastomeric polymers for safety reasons.
Quantifying the risk
Regulators in both the United States and the European Union have now begun to apply a standard risk level that manufacturers can pair with their respective benefit levels for new products. The tolerable risk level for the genotoxic impurities, has been agreed as an excess negative outcome (cancer) of 1 in 100,000 cases. Derivation of a standard tolerable risk level for unknown leachables was one of the desired outcomes of a research project sponsored by the Product Quality Research Institute (PQRI). By comparing negative outcomes from multiple toxicological databases, the toxicologists on the orally Inhaled and Nasal Drug Product PQRI working group derived a common Safety Concern Threshold (SCT) of 0.15µg per day for any exposure compound. Below the SCT, the risk of any unknown leachable has been proposed to be acceptable. This risk can also be understood as an excess negative outcome of 1 in 1,000,000 cases.
Until these quantitative limits were set, regulators would routinely request tests right down to the limits of analytical instrumentation. Analytical capabilities have been evolving to ever lower levels over the past couple of decades, and while this has provided an abundance of information, there is no additional safety margin without the availability of thresholds such as the SCT. Even with these thresholds in hand, formulation compatibility must always be assessed on a case-by-case basis, particularly for biologics. As new and more potent compounds and therapies are introduced to patients, there is a real possibility that lower levels of leachables might cause problems in a formulation. Fortunately, the introduction of risk-based thresholds has provided a level of control of the risks of direct patient exposure that can be measured and, if necessary, managed.
About the Author
Thomas Feinberg
Ph.D.
Sign up for our eNewsletters
Get the latest news and updates