Applying Single-Use Efficiencies to Room-to-Room Fluid Transfer
For biopharma manufacturers, the challenge is clear: quickly and efficiently transfer materials aseptically from one physical area to another, while minimizing the risk of contamination and maximizing efficiency and cost effectiveness. This is especially true for cGMP facilities adapting single-use technology as an operating platform over traditional stainless-steel piping systems. A clear solution to this challenge, however, is more elusive.
TRADITIONAL OPERATIONS
Hard piping transfer lines require intensive, costly, time-consuming cleaning and validation, with a potential negative impact on productivity and manufacturing flexibility. Hard piping is also challenging to expand or upgrade, involving the task of installing each port through a bulkhead. Putting multiple lines of tubing through a standard single-tube port without breaching isolation can be an engineering and maintenance headache, and each new connection adds another potential trouble spot. Lastly, aseptic cleaning involves water consumption and the frequent use of specialized chemicals, which may have a significant environmental impact.
Using tanks and totes for physical transfer avoids some of these problems but generally replaces those problems with others — the risk of leaks and damage, for example, as well as the chore of attempting to wipe down or clean a filled bag inside a mobile tote bin. Tank and tote-based systems are also labor intensive, vulnerable to human error and can be difficult to scale up efficiently.
Traditionally, biopharma manufacturers have accepted the time, labor and monetary costs of FDA-compliant aseptic fluid transfer across isolation barriers using dedicated hard piping, multiple flow-path transfer panels or movable tanks. Manufacturers have had to recognize that downtime is inevitable when ensuring aseptic hard piping transfer — they cannot move product through piping and clean the piping at the same time — and that lost production is an ever-present risk with tank-based systems.
At the same time, manufacturers have accepted the idea that moving multiple lines of fluid across an isolation barrier, via a single port, is difficult and an invitation to trouble, as is rapidly transferring large fluid volumes. Finally, manufacturers have found it challenging to develop systems that are appropriate for a wide variety of clean zone environments.
A WELCOME ALTERNATIVE
The development of single-use components for material transfer has given biopharma manufacturers a welcome alternative to maintenance-intensive hard piping systems or transfer using tanks or totes. Single-use components support environmental initiatives, lean manufacturing principles and cost effectiveness, while ensuring aseptic material transfer.
Figure 1. Single-use systems allow aseptic transfer of fluids through walls and floors via portals as shown.
The concept behind single-use fluid transfer tubing is essentially what the term itself implies: After each transfer operation, the tubing components are discarded and replaced with new, sterile tubing. This ensures that materials are always flowing through an aseptic environment, protecting the biopharma materials from contamination risk while eliminating the need for time-consuming and resource-intensive cleaning and sterilization. Single-use components reduce or eliminate the need for specialty cleaning chemicals, which must be stored and handled as dictated by MSDS documentation.It’s well known that single-use components can help a facility increase its flexibility and sprint capacity to respond to or anticipate changes to production systems. That’s because single-use components are designed to be disassembled quickly and easily for rapid discard and are also convenient to install and configure.
As a result, single-use systems are far simpler to modify, expand or relocate than any dedicated hard-piping system. The ideal single-use system can be installed and taken down by facility staff with minimal training or experience and no specialized tools because intuitive and straightforward connections and seals are involved.
High-quality single-use components present manufacturers with a choice of materials to work with: platinum-cured silicone or TPE (thermoplastic elastomer). Both materials are designed to be discarded after only one aseptic pass-through of biopharma product between clean rooms. Additionally, the materials have undergone extensive physical, chemical and biological testing and meet USP Class VI, FDA, ISO, European Pharmacopoeia and 3-A standards.
BEYOND CLEANLINESS AND SAFETY
The adoption of single-use tubing presents manufacturers with fresh tubing for each material transfer. But the advantages of single-use components go beyond cleanliness and safety. Single-use components can greatly increase flexibility and the ability of manufacturers to anticipate changing needs. For example, dedicated single-use manifold assemblies are ideal for adding multiple fluid flow lines through a single port without compromising isolation barrier integrity.
This eliminates the need for ports with its multiple moving parts while minimizing the maintenance that would otherwise be required to ensure the integrity of an in-wall, pass-through system. Unlike hard piping, single-use components require no welding, and a facility’s staff can install or reconfigure a single-use system usually with minimal training.
Ideally, single-use systems permit the aseptic transfer of fluids through walls or floors regardless of thicknesses and will accommodate multiple lines of fluid through a single wall portal. Purge ports can be incorporated into single-use system portals to reduce the risk of cross-contamination. Purge ports introduce filtered air to create a positive flow through the portal. The system should be able to incorporate purge ports within a single portal along with multiple tubing apertures.
A dependable material for portal assemblies, end caps and hygienic union clamps has proven to be 316L stainless steel, which one single-use manufacturer uses to fabricate all metal components involved in its pass-through system. For the tubing itself, unreinforced, platinum-cured silicone tubing is highly effective, versatile and gamma stable. The low-volatile material is cleanroom produced, undergoes comprehensive testing, and meets all relevant standards for critical applications in biopharma, nutraceutical, cosmetic and food manufacturing facilities.
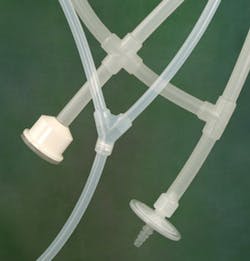
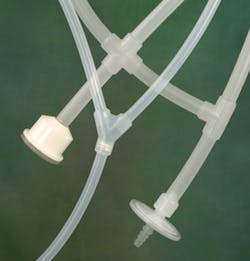
Figure 2. Single-use tubing components require no welding, and the myriad connectors available support its flexibility and adaptability.
TPE tubing for biopharma operations is also available, addressing the need for gamma stable, weldable and heat-sealable transfer tubing without silicone oils. The ideal biopharma grade TPE tubing will maintain its physical properties through sterilization processes, resist kinking in confined spaces, remain translucent for visible product flow and maintain its material integrity with no gummy residue.The ability to create single-use molded manifold assemblies is also crucial to efficient and reliable fluid transfer. Single-use assemblies are well suited to sampling and storage applications; facilities should be able to choose between platinum-cured silicone and biopharma grade TPE. Single-use systems should also offer a choice between aseptic or open connector configurations, single- or double-isolation barriers and user process adaptability.
Sizing is another area in which a range of options is important. The variety of potential applications and processes means that users should be able to choose from an equally wide range of standard tubing sizes, from inside diameters as small as 0.03” up to 1” or more.
The greatest flexibility would be attained with portal sizes from 2” to 12” available as standard and custom fabrication of larger sizes to extend the spectrum of applications for single-use tubing.
While the right single-use transfer system for any facility will provide secure and efficient material flow, many manufacturers would probably say that the flexibility of single-use systems has been equally valuable in their applications. The ability to install, disassemble or modify a single-use pass-through system in minutes, without specialized tools or techniques, adds enormous value to the system and helps a facility become virtually “future proof” — able to adapt quickly to change. One system, for example, allows users to both install and disassemble a pass-through assembly in a few straightforward steps. The changeover quickly makes the facility ready for a new direction in its processes — much more quickly than an equivalent change could be made to a hard-piping or a tank system.
EXPECT FLEXIBILITY
Wall pass-through single-use systems for fluid transfer are well-suited to today’s fast-changing biopharma industry. Single-use components provide an aseptic environment for each process without costly and cumbersome sterilization and validation procedures required with hard piping or other traditional transfer systems. Single-use requires no CIP chemicals to use and dispose of and no extensive use of water. It’s leaner than tank and tote transfer and minimizes the use of resources — human, financial and natural — offering a smaller carbon footprint. Finally, single-use systems offer unsurpassed flexibility in dealing with process changes and can retrofit easily into existing operations. Exploring single-use concepts can lead to changes that make any facility’s processes leaner and more adaptable.