Maximum and Minimum Loads in Steam Sterilization
Steam sterilization is a critical process in the manufacture of many pharmaceutical and medical device products. Because of its importance and wide usage it receives a great deal of attention from both practitioners and regulators. Despite this focus, there are aspects relating to load size that prove troublesome, and can cause difficulty during sterilization cycle development, and validation. Regulators will routinely query users on their means for validation of varying load sizes in both pre-approval and routing inspection. The regulatory expectation is that the user has validated fixed and invariable load patterns, and thus load sizes for all sterilization processes.¹,² This is expected for both parts (porous) and terminal (non-porous) loads. While defined loads has been used by some firms, their use constrains operational flexibility and many industrial users have sought more flexible, and efficient means. Depending upon the details of the individual sterilization processes, their ability to defend their practices relating to load size variation may not be adequate.
It has been common practice to utilize maximum loads as "worst case" demonstrations of sterilization lethality and assume that smaller size loads will be appropriately sterilized. The assumption is that the larger size, number, and mass of the maximum load represents a worst case and challenges the sterilization process sufficiently during validation that the minimum load (and all intermediate size loads) can be considered acceptable by default. Depending upon the manner in which the sterilizer is controlled during the process, this may or may not be true. Varying air removal, and/or differences in heating /cooling rate as is possible with varying load size, can result in process differences that can result in reduced cycle efficacy.
This article will address the effect of load size on both parts (porous load) and terminal sterilization processes. The specific means to address load size differ between the sterilization processes; however the general approach to follow is similar. Using the content provided herein, the practitioner can establish their sterilization processes for either porous or non-porous loads with confidence that they can meet regulatory expectations, while maximizing operational flexibility.
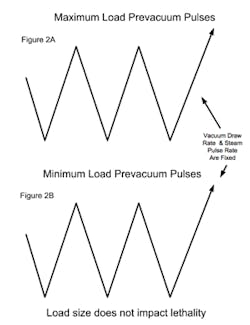
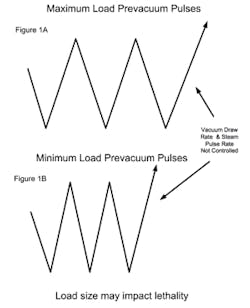
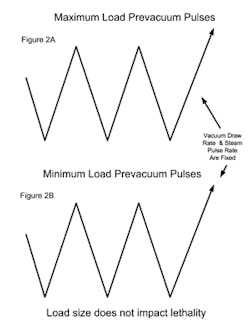
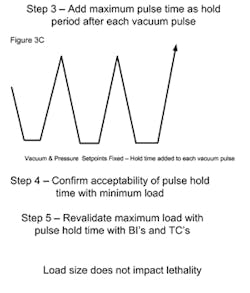
PARTS (POROUS) CYCLESA major consideration with porous loads is that air must be efficiently removed from the load. As the maximum load typically has many wrapped items, the rate at which air can be removed can be influenced by the load size. Air present within wrapped items must diffuse through the wrapping, whereas air removal from outside the load has no restrictions and is rapidly removed (see Figure 1A). When a minimum load is processed, the amount of internal air is reduced and it is possible that air within the load items may not be adequately removed when the targeted vacuum level is attained (see Figure 1B). The presence of air retained within the load item can result in the minimum load receiving less lethality than necessary for its reliable sterilization. The means to address this potential problem vary with the sterilizer control system and its sophistication. Note that in all of the scenarios the number of vacuum pulses would be the same and defined by what is necessary to meet any defined equilibration time requirements.
TERMINAL STERILIZATION CYCLES
The sterilization of liquid filled aqueous containers represents a more challenging situation in that not only is there a minimum lethality expectation; the materials in the load must also not be over-processed. The means to assure consistency across load size is often facilitated because terminal sterilizers are ordinarily equipped with more sophisticated control systems allowing for the regulation of heating and cooling rates. Before commencing the effort, the lethality required to sterilize the filled containers must be chosen from container mapping studies and information on the expected bioburden resistance and population. This calculation will typically include some added margin of safety to ease routine operation.
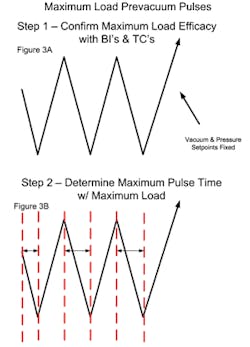
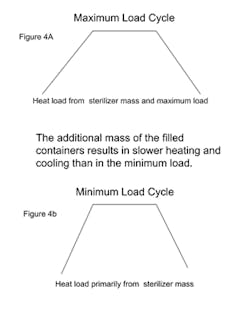
Replicate mapping studies with the maximum load are performed next to identify the minimum and maximum lethality locations (these are more commonly a region or zone than an individual container). During these studies the heating and cooling rates should be set at approximately 90% of the equipment’s full capability (see Figure 4A). This value is selected to ensure controllability while not extending the overall cycle appreciably.
CONCLUSION
The methods outlined should enable the practitioner to efficiently and confidently validate maximum and minimum loads undergoing steam sterilization with a fuller understanding of the control elements that will assure consistency of efficacy. The control suggestions indicated allow for greater flexibility in operation while maintaining the required lethality and product stability which are essential to regulatory compliance
[1] FDA, Guideline - Guidance for Industry for the Submission of Documentation for Sterilization Process Validation in Applications for Human & Veterinary Drug Products, 1994.
[2] EMA, Annex 1, Sterile Medicinal Products, 2008.