Pharmaceutical and biotechnology companies across the world are therefore keen to expand their CGT & RM portfolios into new geographies and grow their impact. Similarly, many countries have sought to create enabling environments to support faster routes to market for safe and effective CGT & RMs. Among these countries, Japan has emerged as a go-to location, thanks to its attractive patient population and unique, highly enabling CGT & RM regulations.
However, expanding a CGT & RM therapy portfolio into a foreign country such as Japan can be challenging. Companies face a dizzying array of considerations and many manufacturing choices —and picking the right one involves a careful look into each option.
Weighing up the options
Companies looking to commercialize a CGT & RM product in Japan have three major manufacturing options, each with important advantages and disadvantages. Choosing the right option depends on carefully weighing these in light of a wealth of factors, including the nature of the therapy, available resources (both human and technical), the company’s geographical proximity to Japan, as well as its long-term commercial vision for the region.
Option 1: Local manufacture before shipment to Japan: simplified manufacturing, but logistical complexities
One approach that companies can take is to manufacture their therapies locally — either in-house or with local CDMO support — before shipping them to Japan.
Local manufacture in your home country has a range of advantages for CGT & RM manufacturers. For example, and perhaps most importantly, local manufacture means companies can avoid technology transfer to a foreign country. Technology transfer, even internally, is difficult enough when it comes to CGT & RMs, owing to their inherently complex and difficult-to-scale processes. But technology transfer to another company, in a different country, adds additional challenges into the mix — not being able to rely on your usual project management and governance structures, and trickier communications, to name just a few.
Moreover, manufacturing locally enables centralized manufacturing, along with all the benefits that entails — standardization of production and quality for more consistent products, more efficient resource use, and the ability to leverage economies of scale (with all manufacturing operations under one roof, companies get faster and more efficient processes, which means a lower cost-per-unit). The control afforded by centralized manufacturing also means companies can better adapt to rapid market changes.
Local manufacture, then, is an attractive option for several reasons. But it has some significant disadvantages, too.
The most important is that long distance shipping of delicate products for time-critical use is logistically complex and very expensive (Figure 1). For success, manufacturers must control for many potential disruption points across the supply chain and ensure clear and continual communication with stakeholders across courier companies, clinical sites, and manufacturing facilities. Since the process is so complex, companies also need a thorough and flexible logistics strategy in place well ahead of accepting any product orders.
Long-distance shipping poses even greater challenges when it comes to autologous therapies, as the time considerations are particularly pressing. Even if local manufacture and international transport of autologous CGT & RM products is possible, the risk of product degradation during transit is high. To mitigate the risk and make this approach feasible at all, companies would need, at the very least, a highly reliable cryopreservation system (assuming the therapy is suitable for cryopreservation); smooth, rapid, and well-managed shipping; quick yet well-controlled dispatch testing; and rapid acceptance testing upon arrival in Japan (for autologous therapies that aren’t suitable for cryopreservation, the transport process is even more complex.)
Option 2: Setting up site in Japan: more logistical control, but resource and knowledge intensive
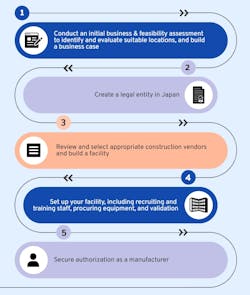
Alternatively, manufacturers looking to commercialize their therapies in Japan could build their own development and manufacturing infrastructure in the region (Figure 2).
Unsurprisingly, having manufacturing facilities in the same country as patients offers attractive logistical benefits, as well. For a start, companies can avoid overseas transportation of products, needing to focus only on local distribution, which simplifies and streamlines the process. For the same reason, manufacturing CGT&RMs in Japan is well suited to both autologous and allogeneic therapies.
That said, building infrastructure from scratch in Japan — or anywhere else, in fact— involves large upfront costs and long-term, resource-intensive management. For example, Takeda’s recent 100 billion JPY investment in a Japanese plasma-derived therapy manufacturing facility, while not a CGT & RM plant, serves as a clear illustration of the costs involved in building advanced facilities. It’s no surprise, then, that self-building in Japan is typically only feasible for the largest and most well-equipped pharmaceutical and biotechnology companies.
Being financially well-equipped won’t be enough to ensure self-building success, however. Companies must be able to meet Japan’s complex regulatory requirements for constructing and maintaining a Japanese manufacturing site, involving becoming an authorized manufacturer, regulatory inspections, and passing unannounced audits.
Finally, whatever the location, creating a new manufacturing facility from scratch demands effective communication and smooth management. But building a new manufacturing facility in another country is an order of magnitude more complicated still. When it comes to Japan in particular, companies must be able to navigate large language and cultural differences to succeed.
Option 3: Partnering with a Japan-based CDMO: the best of both worlds if you pick the right partner
A third option for developing and manufacturing CGT & RM products for the Japanese market is to partner with a CDMO based in the region.
Partnering with a Japan-based CDMO combines many of the advantages of local manufacture followed by international shipping and building Japanese facilities. For instance, as with local manufacture and shipping, companies working with a Japan-based CDMO won’t need to invest heavily upfront for manufacturing infrastructure, or grapple with Japan’s regulations for building and maintaining a facility in Japan.
Partnering with a Japan-based CDMO is also suitable for both autologous and allogenic therapies, and, critically, offers significant logistical benefits, especially for autologous therapies that demand swift and meticulously controlled and tracked distribution. In fact, because of the benefits of close-to-patient manufacturing, some CDMOs seek to build facilities as close to medical centers as possible, to further simplify the logistics of material supply and product distribution.
Working with a CDMO also offers a critical advantage beyond those offered by other options — the flexibility to develop and manufacture just one therapy in Japan. Companies no longer need a full pipeline of CGT and regenerative candidates to make product manufacture and commercialization in Japan economically feasible.
Success, however, depends heavily on the choice of CDMO partner, as not all Japan-based CDMOs were created equal. Several different Japan-based CDMOs operate in the CGT & RM space, and they vary greatly in terms of their breadth and depth of expertise.
Making the right decision: What enables a successful CDMO partnership in Japan?
With so much at stake and given that working with a Japan-based CDMO involves complex foreign technology transfer, CDMO choice is critical. To maximize chance of success, companies should look for the following capabilities in their CDMO partner:
Regulatory understanding and expertise
Japan’s regulations are some of the most enabling in the world, helping companies accelerate the delivery of transformative products to patients. But for companies that aren’t familiar with Japan’s regulations, they pose a potential roadblock to development and manufacturing success. That’s why manufacturers should prioritize CDMOs with deep and extensive regulatory expertise and established relationships with regulatory authorities. That way, they can grasp regulatory expectations early, and secure early, frequent consultation with regulatory authorities to mitigate risk and unearth any applicable regulatory and financial privileges.
Manufacturing know-how and tech transfer expertise
Manufacturing CGT & regenerative products is inherently difficult and risky. Manufacturers must contend with sensitive and variable starting materials, as well as complex, non-standardized, and hard-to-scale manual processes. Transferring such processes across the world is therefore a delicate task, where, in inexperienced hands, lots can go wrong.
It only makes sense, then, to select a CDMO partner who has extensive expertise and experience in a wide range of CGT & RM manufacturing technologies and approaches, as well as a track record of successful process development and complex global technology transfer projects.
Strong logistics networks
Even though manufacturing a CGT&RM product within Japan significantly simplifies material and product transportation, it can still be challenging to get right. Careful and efficient management of logistics is essential to ensure swift delivery and maintain the exacting environmental conditions that these therapies demand. Without it, you face delivering a poorer quality product to patients, or no product at all, given it may need to be discarded.
There are many logistics companies in Japan. But, critically, not all CDMOs will be able to partner with and manage them to ensure smooth and swift CGT&RM delivery. Given the risks, it is important to ensure that your CDMO partner can — and that it has already done so, ideally through a track record of successfully distributing delicate CGT&RMs across Japan.
A strategic location
Several regions of Japan have been specifically developed to maximize the potential and efficiency of CGT&RM development, manufacture and commercialization. Take, for example, the Kashiwanoha platform, one of eight bio-innovation hubs within the Greater Tokyo Biotech (GTB) community, an industry-academia-government network created to strengthen and expand the biotechnology sector. The Kashiwanoha hub offers easy access to a range of advanced facilities and infrastructure to support innovative therapies.
Choosing a partner that is deeply embedded in one of these platforms could significantly benefit your manufacturing and commercialization program, unlocking efficiencies and putting you closer to the knowledge, technologies, and expertise that can drive success.
A one-stop-shop service offering
Choosing a CDMO partner that offers end-to-end CGT&RM services can simplify your program management by minimizing the number of partners you need to work with. What’s more, a CDMO with a breadth of knowledge and experience across the CGT&RM value chain ensures that they will be able to optimize each stage for efficiencies at subsequent stages, enabling greater chances of success. As such, companies should prioritize full service CDMO partners for their CGT&RM manufacturing and commercialization.
A history of success
Having a track record of success can arm a company with problem-solving and efficiency-identifying abilities that would be hard to get in other ways. To grapple with the complexity of CGT&RM manufacture, companies should therefore seek CDMO partners that have trodden the path to CGT&RM success many times already.
While the CGT&RM sector is relatively new, some Japanese CDMOs have already brought as many as five CGT&RMs to market in Japan, and have supported with tens of complex manufacturing technology transfer programs.
Open the door to Japan’s promising CGT&RM market
Excitement around CGT&RMs is building across the globe. But nowhere is this excitement greater than in Japan — a country committed to accelerating the delivery of advanced CGT&RM products to patients in need.
Pharmaceutical and biotechnology companies have recognized Japan’s potential and are now exploring their options for CGT&RM portfolio expansion in the region.
But with many manufacturing options available — and much at stake — companies must carefully weigh the advantages and disadvantages of each approach.
Partnering with a Japan-based CDMO could be the ideal option to take advantage of Japan’s attractive CGT&RM market, but only if companies select a competent CDMO with the skills, technologies, and experience to level the path to success.