Drug development continues to change dramatically (see Data Use and Misuse, below). As a result, the amount of information that pharmaceutical companies generate and collect during drug development doubles every five years. Unfortunately, only 10% of this information is ever leveraged to improve overall competitiveness and compliance [1]. We decided to find out why, in a survey (Pharmaceutical Manufacturing, April 2005, p. 73) designed to assess the current state of information and knowledge management during pharmaceutical and biopharmaceutical process development.
The survey (see Demographics and Process Development Growth Trends, below) benchmarked each respondent against an ideal, in which business and information processes are characterized by:
- Visibility
- Traceability
- Accessibility
- Leveragability
- Structured Data
In brief, drug development professionals know where they need to go to optimize data management. They also have a clear idea of what they need to perform their jobs better. However, the organizations in which they operate often fail to see the big picture. They don’t integrate or align business and information processes, or create the required to transfer business, science and compliance data across development silos.
Data transparency
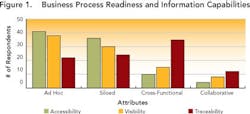
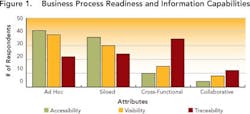
Since data transparency is the prime prerequisite for knowledge management, the survey first assessed how visible, accessible and traceable information was in each respondent’s organization. Results indicate that most drug development organizations need to get a better handle on the data they generate. As Figure 1 (below) shows, most drug development organizations — regardless of size — are working with ad hoc or siloed processes, in which data are not being captured consistently. This results in fire-fighting when issues come up, as well as “tribal data,” in which individuals store most of their data on individual desktops or servers.