Tangential flow filtration is old school. It’s been used in a variety of industries for decades—take blood and food processing, for example—and has become standard in biopharmaceuticals as an efficient and cost-effective means of separation. But you can’t really say that tangential flow filtration (TFF) has found its niche in biopharma, because as technologies change, so do applications.
The niche shifts, expands and contracts. Biopharmaceutical developers and manufacturers are using TFF in place of, or in tandem with, ultrafiltration, microfiltration, and diafiltration. It has found favor as a means of concentrating sample solutions (large and small), fractionating molecules, harvesting cells, and clarifying fermentation broths or cell lysates. In some biomanufacturing plants, tangential flow is replacing the need for material centrifugation.
North Carolina-based NCSRT, Inc. develops and optimizes filtration systems for a number of smaller and larger manufacturers, and lately the majority of its business is in patented two-step TFF systems, which offer the promise of higher yields, fewer purification steps and shorter operating time. “I used to do 40-liter batches of Epstein-Barr virus in a centrifuge for one month, and get a 10% yield,” founder and chief technology officer Hank Kopf says. “Now [through tangential flow filtration], I’m getting 90% of the virus and eliminating contaminants.”
NCSRT has teamed with Winnipeg’s Viventia Biotech to document the advantages of its SmartFlow TFF over centrifugation for clarifying and recovering Viventia’s Phase I/II VB6-845 antigen-binding fragment (Fab) (See Figure 1 below). It’s one example of how TFF is making inroads into new areas of bioprocessing. The adoption of TFF is not wholesale, however, notes Kent Iverson, an independent biologics CMC consultant. Take clarification of cell culture broth.
TFF is actually being replaced by centrifugation in many plants, and by direct flow “dead-end” filters in others—they’re often simpler and cheaper, Iverson says. That’s not to say that there isn’t a trend developing here. “The tangential flow filtration market will continue to grow,” Iverson says, especially as a means of ultrafiltration. In addition, intriguing TFF-based processes will continue to crop up in as-of-yet untested areas (box at right).
Killer Apps for TFF
|
A Tested Technology
As its name implies, tangential flow filtration involves a feedstream which flows parallel to the filtration membrane surface. Permeate passes through the membrane, while retentate returns to the original feed tank (Figure 1). The cross flow (TFF is also known as cross flow filtration) means less molecule buildup and fouling of the membrane surface. Applications depend upon multiple factors: among them, the nature of the feed material (e.g., the viscosity or density of cells), the geometry of the feed channel flow path, feed velocity, and filter material, size and configuration.
Contract R&D and manufacturing outfits, which tend to have greater product turnover and a willingness to showcase novel technologies, deserve credit for testing and popularizing TFF of late, NCSRT’s Kopf says. While single-use or disposable TFF systems are gaining traction in labs and pilot plants—with membranes, housing, valves and tubing all capable of being disposed of rather than cleaned and reused (more on this later)—most manufacturers are highly invested in their tried-and-trusted production platforms. And they’re hesitant to devote the time and effort required for the thorough extractable and leachable studies needed to qualify disposable materials.
What’s more, technology for reusable TFF continues to advance, whether the system is based upon flat-sheet cassettes or cylindrical hollow-fiber modules. Improved cleanability and flexibility are two key developments. Sartorius Stedim’s Sartocon series of cassettes, for example, now have steam-in-place capability for aseptic processing, says Christian Manzke, the company’s director of purification technologies. For non-sterile applications, the company’s Hydrosart regenerated cellulose filters can withstand caustics ranging in pH from 1 to 14.
Viventia Goes with the (Cross) Flow
Winnipeg’s Viventia Biotech is a privately held developer of MAb-based oncology drugs. The manufacturer’s VB6-845, currently in Phase I/II clinical trials for the treatment of solid tumors, is a cytotoxic therapeutic produced in E. Coli and is purified from the culture broth supernatant. Viventia evaluated different means of clarifying and recovering the VB6-845 antigen-binding fragment (Fab), and thus compared three methods: stacked disc centrifugation followed by microfiltration; tubular bowl centrifugation followed by microfiltration; and tangential flow filtration using SmartFlow TFF, a proprietary system of simultaneous microfiltration and ultrafiltration developed by North Carolina-based NCSRT, Inc.
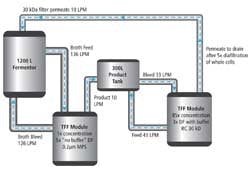
Results showed Fab VB6-845 recoveries of less than 40% for each of the centrifugation methods, while recovery based on the TFF systems was 85.7%. The figure shown here represents a hypothetical 1,200-liter operation:
Figure 1. Click for larger image.
For centrifugation, 38 1200-liter runs would be required to produce 1 kg of product, compared to just 12 batches for the simultaneous SmartFlow TFF system. By increasing diafiltration volumes, Viventia found that it could further reduce the number of fermentor runs needed to gain 1 kg, and projected that it could complete a 1200-liter batch in less than 8 hours.
This application of SmartFlow TFF was designed to simultaneously isolate a molecule and concentrate the product, and relies upon two filtration skids. Product slurry in the harvest tank passes through the first TFF skid, isolating the product fragments. This isolation step can use either a microfiltration membrane or ultrafiltration membrane with a high-molecular-weight cutoff to filter out unwanted cell debris.
The product is then passed through the second skid and concentrated via an ultrafiltration membrane. The permeate from the second skid is pumped back to the original harvest tank and used as diafiltration buffer in the ongoing process.
Customers are asking for, and getting, more compact systems, says Thomas Scholz, director of marketing for TFF at Pall Life Sciences. The geometries of TFF cassettes like Pall’s T-Series line have advantages over older models such as hollow fiber membranes in that lower velocities are required to keep particles from fouling the filter membrane, and thus lower pump capacity is required, Scholz says. Smaller, more compact systems allow for greater efficiencies, less protein loss and thus cost savings.
Another advancement in cassette TFF is the use of harder, rigid polyurethane frames that are resistant to overtorquing and damage from continued use. Pall has extended the use of polyurethane to its entire T-series line of cassettes, with filtration areas ranging from 200 cm2 to 2.5 m2. The cassettes can be stacked for process scaleup up to 20,000 L, the company says. Since the cassettes have the same path length and materials regardless of capacity, drug manufacturers are employing them from early process development through full production, getting their filter experimentation and extractable analyses out of the way in the first stages of development. Millipore continues to see broader adoption of its Pellicon 3 thermoplastic TFF cassettes.
The modules are self-contained—the gaskets are built in—and can be loaded and stacked to allow for linear scale-up, so that process parameters in the lab can mimic those that will be used on the plant floor. Pellicon 3 cassettes are molded and require no adhesives, which eliminates concerns of extractable contamination and leaking, and allows for higher operating pressures—up to 80 psi. Pellicon 3 cassettes range in size from 88 cm2 to 1.14 m2, says product manager Dan Dussault. Indeed, the ability to scale up, or scale down, without changing basic flow properties is facilitating process development and consistency from bench- to productionscale, says Gerard Gach, head of global product marketing for filtration, Ready-to-Process, and WAVE technologies at GE Life Sciences.
Customers can scale linearly from lab through process development to production volume without changing flow paths of their cross flow filters, whether cassette or hollow-fiber. Vendors have also increased the software and services they offer. For GE, that means offering the tools and software needed to screen multiple filter types automatically, as well as a web-based tool for selecting the right filter for a given process. The latter, called Filter Brain, allows users to input key process parameters and receive an immediate recommendation as to the proper filter to use.
Charged Membranes
Membrane technology continues to make “incremental” improvements, notes Iverson. Choices for TFF boil down to either regenerated cellulose (RC) or polyether sulfone (PES). While filter choice is always applicationdependent, there is a trend toward RC because of its more hydrophilic character and lower binding capacity, Scholz says. PES filters are known to be more robust and hold up better under rigid cleaning. NCSRT’s Kopf has seen a vast increase in filter options over the past few years.
There are greater combinations of pore sizes, membrane-protein bonding capabilities, and the ability to positively or negatively charge the membrane, all of which allow companies to tweak their filtration characteristics to suit a given process step. GE alone offers 700 variations of its hollowfiber models, and 50 of its TFF cassettes, Gach notes. The most significant advancements in tangential flow in the past few years have to do with membranes and their chemistry, says Dr. Michael Meagher, director of the University of Nebraska-Lincoln’s Biological Process Development Facility.
Like Kopf, Meagher marvels at the robustness, pore distributions and control of pore chemistry of today’s membranes. High-performance tangential flow filtration (HPTFF) lies at the cutting edge of these advances, as a means of enabling concentration, purification and formulation in a single unit operation. HPTFF uses a charged membrane that allows manufacturers to separate product based on both molecule size and charge.
Interest in the technique has been spurred by Genentech researchers, who have run feedstreams of recombinant proteins expressed in E. coli and achieved a 10-fold removal of host cell proteins with an overall process yield of 98%. Compared to a more conventional purification scheme, the process eliminated one chromatography step and a 12% improvement in yield. Beyond HPTFF, the future will see further increases in membrane capabilities for all filtration processes, says Dussault of Millipore. Manufacturers will expect tighter specifications and greater consistency to match their increasingly specific processing needs.
The Disposable Dilemma
While technology advances have allowed reusable TFF systems to remain standard, single-use (i.e., disposable) components are gaining traction. Disposable filter cassettes, as well as bags, adaptors, tubing and other filtration system components, are now commonplace.
Major filtration vendors are racing to offer entire off-the-shelf disposable tangential flow systems—Sartorius Stedim, for example, expects to have completely disposable tangential flow filtration systems for lab- and pilot-scale late this year or early next. Pilot plants and contract manufacturers with high product turnover who seek to minimize the risk of cross-contamination, limit cleaning and validation time and reduce water consumption are naturally gravitating towards single-use systems.
Disposables allow startups to conduct research with minimal investment in equipment. “They’re awesome,” says Nebraska’s Meagher of disposable systems. His operation is what he calls an “academic CMO on steroids,” and he speaks in particular of essentially having contained, plug-and-play TFF systems. GE plays up this aspect of its technologies, billing them as “Ready-to- Process”—presanitized, preflushed, and preconditioned, Gach says.
The technology is there for singleuse to perform at production scale, says Pall’s Scholz. Pall’s Kleenpak TFF capsules typically run at process of volumes in the neighborhood of 200 liters, but can be manifolded to accommodate larger processes. Millipore’s disposable Pellicon TFF cartridges were designed with clinical manufacturing in mind, but can also be stacked for greater production volumes.
One person not entirely sold on the disposable movement is Kent Iverson. “The disposable trend is real and desirable,” he says. But in most cases the cost of disposable TFF components is much greater than that of reusable. “The whole cost analysis of disposables versus reusables has been clouded by the fact that the people doing the analysis are selling the products,” he says. It’s expensive to keep throwing filters away, he notes. One reason that contract manufacturers like them is that they can pass the costs on to the client. “The pendulum will swing back towards reusables as methods of accounting mature,” Iverson says.