Pharmaceutical Process Control: Is the Great Divide Growing?
Five years ago this month, FDA published its 21st Century GMP’s guidance document, challenging pharmaceutical manufacturers to use modern risk management and manufacturing methods to improve product quality and boost efficiency.
Central to the guidance is the idea of continuous quality improvement, and it specifically mentions process analytical technology (PAT) and even continuous manufacturing.
One might think that these concepts have become part of the fabric of drug manufacturing today, but there are signs that adoption of modern manufacturing practices continues to be uneven. Is there a divide separating adopters from a core group of non-adopters? In a challenging economic climate, is “failing to act” becoming a strategy in and of itself?
This article summarizes results of our fourth informal reader survey on pharmaceutical process control and automation, in light of 21st Century GMPs and other FDA guidance documents. A subsequent article will appear on PharmaManufacturing.com, to discuss these results.
Although results may not be statistically significant, they serve to highlight the broadest issues and trends and how they appear to be changing.
The number of respondents this year varied, depending on the questions posed. Up to 95 industry professionals responded to questions about PAT and QbD adoption, and overall alignment of IT and automation with use of technology, where 58 to 67 replied to more detailed questions involving process control and manufacturing practice.
Responses reflect practices at large and small pharmaceutical companies alike, with roughly one-third coming from big pharma and big biotech companies. However, this year’s survey included more responses from smaller contract research and manufacturing organizations and professionals involved in back-end drug development efforts.
Aligning Technology with Operational Excellence Goals
This year, of 95 respondents, 15% say they have aligned automation and IT to continuous quality improvement efforts such as Lean and Six Sigma. 19% describe such alignment as a top priority, while 38% say progress is being made (Figure 1).
However, 28% say there isn’t really any alignment, an increase from last year’s 22%, but significantly less than the 31% who described their facilities that way in 2007’s survey.
Improving data access continues to be a top goal for many manufacturers, the survey suggests. For most of the 78 professionals who replied to this question this year, the most pressing priority is developing trending data for equipment and batches. Rounding out the list of “extremely important” goals are integrating manufacturing floor to ERP and integrating field-level sensors to the plant floor.
Few respondents this year say they have achieved full plant floor to enterprise integration; 6% say they’re integrated while 12% say they’re almost there. “It’s still a long way off,” said 17%, while 9% say that integration is not a goal for their organizations.
Last year, 9% described their organizations as integrated, while 29% said they were “almost there.” In 2007, these figures were 7% and 14%.
MES adoption appears to have stalled, if this year’s results are any indication. Of 77 respondents, 42% are not using MES, 30% are and another 26% are evaluating for the future (Figure 2). Obstacles cited were difficulty of integration and the time and cost of implementation (Figure 3)
Last year, 29% weren’t using MES, 22% were and 46% were planning to install.
PAT Backlash?
PAT and QbD adoption continue to increase, but progress does not appear to be steady, and a pocket of resistance to both concepts may even be growing.
Consider respondents who say they aren’t doing PAT or QbD and have no plans to do either. In 2006, that number for PAT was 20% of 75 reader respondents. The following year, when QbD was folded into the question, it had increased to 22% of 67 respondents. In 2008, 29% of 62 respondents said they were not interested in exploring either PAT or QbD.
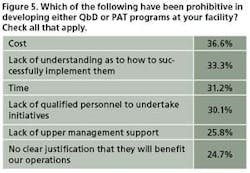
This year, 31% of 93 respondents said they were not interested in launching either PAT or QbD programs (Figure 4). Cost, lack of understanding as to how to implement these concepts, and lack of time were cited as the major obstacles to launching PAT or QbD projects (Figure 5).
Pharma Process Understanding
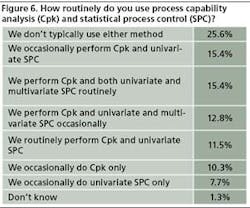
In line with previous years, relatively few respondents say they are using process capability analysis or statistical process control in any systematic way. This year, 26% of 78 respondents say they don’t typically use either method, while roughly 15% use both CpK and uni- and multivariate SPC regularly. Roughly 10% use CpK only, and 7% use univariate SPC only (Figure 6)
Last year, 31% of respondents said they didn’t use CpK or SPC, and in 2007, the number was 28%.
Alarm Management
Managing alarms, a key to achieving Jidoka is becoming more of a priority than it was a few years ago. In our first survey, 35% of respondents didn’t have an alarm management strategy in place and another 10% didn’t particularly care about the issue. This year, 43% of 68 respondents have an alarm management strategy in place, and 16% are developing one (Figure 7). Wrote one respondent, “There are alarms but they are not handled appropriately and there doesn’t seem to be any inclination to do anything differently.”
Advanced Control
Simulation is playing a more active role in more respondents’ operations. This year, 67% of 58 respondents say they are using simulation and other advanced control, mainly in small and large molecule manufacturing and drug development, while 26% say they are using model predictive control.
45% are using simulation to improve process design while 17% use the tool to help with construction and expansion projects (Figure 8).
Wireless and Robotics on the Edge
Wireless monitoring and control are being adopted by 38% and evaluated by another 34%. Warehouse applications dominate with 17% while process applications are becoming more important (Figure 9).
Robotics is being used mainly in packaging and palletizing; over 28% of 67 respondents say they are using and another 10% plan to use it, 61% of respondents say they have no plans. Hurdles included perceived high costs and uncertain ROI.
Continuous Manufacturing Makes Inroads
One recurring, if muted, theme in many of FDA’s recent guidance documents and in its draft process validation guidance is the potential that continuous processing has to reduce variability and cost.
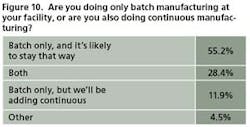
This year, respondents showed a bit more resistance to the idea than in past years, with 55% of 67 saying they have no plans to get into continuous processing. However, 28% already do both and another 12% plan to increase their use of continuous manufacturing (Figure 10).