DOE Improves Throughput in Manufacturing of Key Intermediate
July 24, 2013
7 min read
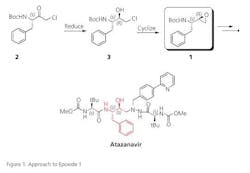
A key intermediate, (2S, 3R)-Epoxide (1) is used in the production of Atazanavir (marketed as Reyataz), an antiretroviral drug used to treat human immunodeficiency virus (HIV). Existing methods of producing epoxide 1 involve the diastereoselective reduction of the amino acid derived ketone 2 followed by cyclization of the intermediate chiral alcohol 3. However, the reported approaches (see Figure 1) suffer from either low selectivity or low throughput and most also utilize hazardous reagents or catalysts. Codexis felt there was potential to improve on these methods by using an isolated ketoreductase (KRED) enzyme to enable a biocatalytic reduction of ketone 2.
Codexis researchers screened its extensive KRED library and found hits with near perfect chiral selectivity. However, throughput of the initial screen was too low for commercial applicability. The researchers improved the performance of the enzyme using directed evolution, and also performed two stages of design of experiments (DOE) to identify and optimize key process variables. The final process conditions provided 99%+ selectivity and throughput 50% above the target level without requiring any hazardous reagents.
The first
Atazanavir was the first protease inhibitor approved for daily dosing and also has lesser effects on the patient’s lipid profile. More recent research has found that the drug can inhibit the growth of brain tumor cells, so the drug is being investigated for anti-cancer applications. Epoxide 1 is a key intermediate in the chemical synthesis of Atazanavir. The diastereoselective reduction of chloroketone is the most challenging step in the production of the epoxide.
One reported approach involves reduction of ketone 2 with hindered hydride reagents. However the chiral selectivity of this approach is suboptimal and upgrade of the diastereomeric purity via recrystallization is required, resulting in significant yield loss. An alternative approach, whole cell bioreduction using a Rhodococcus species, provides good chiral selectivity but with very low substrate loading — which translates to unacceptably low throughput.
The use of KRED-catalyzed reduction is now an established strategy to manufacture chiral secondary alcohols in very high chiral purity. However, with some substrates natural enzymes are not sufficiently active or capable of delivering the product in high enough chiral purity. In such cases, the product requires upgrading, resulting in low yield. Directed evolution technologies have been used to deliver superior enzyme catalysts, including KREDs. The enzyme is optimized to provide high activity and outstanding selectivity for products that previously were produced with poor selectivity or were even inaccessible with natural enzymes. Simultaneously, the catalysts can be engineered to withstand the rigors of a commercial manufacturing environment, allowing them to withstand conditions intolerable for many natural KREDs.
Codexis investigates
Codexis researchers investigated the potential for achieving both high selectivity and high throughput by producing alcohol 2 using an isolated KRED. They screened the company’s extensive KRED library for activity and found 18 hits with 100% selectivity for the desired stereoisomer. However, the initial performance of the screened enzymes suffered from low substrate loading of 3 g/L, high catalyst loading of 5 g/L and conversion of only 30%. The goal was to achieve substrate loading of 100 g/L, catalyst loading of 1 g/L and conversion above 99%. Improvements were made to the enzyme using directed evolution technologies, and the process was developed in conjunction with these efforts.
Trying to improve the process using traditional one-factor-at-a-time (OFAT) experiments would have been expensive and time consuming. The researchers turned to DOE because it is specifically intended to identify interactions between process variables that play a critical role in pharmaceutical manufacturing. This powerful approach makes it possible to identify ideal combinations of factors in far fewer experimental runs than the OFAT approach. DOE varies the values of chosen factors in parallel so it uncovers not just the main effects of each factor but also the interactions between factors.
DOE enables chemists to efficiently define, better understand and optimize factors that are important to yield and robustness, particularly where multiple parameter interactions are involved. The Codexis team uses Design-Expert software from Stat-Ease, to design and analyze DOE experiments. They originally selected the software because it is designed for use by subject matter experts who are not necessarily experts in statistical methods. The software walks users through the process of designing and running the experiment and evaluating the results.
In this case, the team picked the most promising catalyst candidate and performed DOE with the goal of rapidly optimizing the process to achieve these goals. Codexis used Design-Expert software to create a fractional factorial experiment with six factors as shown in Table 1 and four center points for a total of 20 experimental runs. The conversion and chiral selectivity of each run was measured.
The results showed that conversion was strongly dependent on pH and amount of IPA in the aqueous buffer. There was also a significant interaction between these two variables. Changing both variables simultaneously increased conversion more than would be expected from the single variable effects alone. The diastereoselective of the enzyme was unaffected by the variables studied.
A second DOE was used to optimize key factors in the process as determined by the initial DOE (see Figure 3). The second DOE was a fractional factorial experiment with four factors and four center points and a total of 12 runs (see Table 2). The cofactor was set at 0.5 g/L and the concentration at 10 volumes because the initial DOE showed these two factors did not have a significant impact on the results. The reaction was sampled at 24 hours and the conversion and chiral selectivity were measured. The results showed that conversion was still strongly dependent on pH, and temperature also showed an influence. As expected, the conversion was also dependent upon the amount of enzyme charged.
Additional refinements to the process included a further increase of substrate loading, a reduction in IPA loading, and an increase in reaction temperature. The final process conditions were a pH of nine and the use of 10% IPA solvent in buffered water at 45 C. The product was extracted into an organic solvent and clarified to remove any traces of the enzyme.
Codexis researchers also optimized the remainder of the process for producing epoxide 1. They sought a solvent for chloroalcohol extraction that provides telescoped ring closure while being immiscible with water and providing a clean phase split. Rapid and clean conversion is required and the epoxide product should be crystallized from the reaction solvent or in an easy solvent swap. A focused screen of base/solvent combinations was performed. Methyl tertiary butyl ether (MTBE) gave excellent performance so it was selected as the preferred extraction/reaction solvent. Potassium hydroxide (KOH) was found to be a suitable base and given its low cost and ready availability was chosen as the base. The metrics for the final process are shown in Table 3.
The final process begins with reduction of chloroketone with KRED biocatalyst in a mixture of 10% IPA in aqueous buffer at pH of 9 at 45 C. The product is extracted into MTBE and undergoes clarifying filtration. KOH is added and the resulting mixture is cyclized to epoxide. The organic phase is washed with water and undergoes a solvent swap to heptane. Crystallization and filtration yields pure epoxide.
Codexis researchers rapidly developed an efficient catalytic manufacturing process for manufacturing epoxide 1. The catalyst was initially identified from Codexis’ panels of evolved KRED variants and was engineered to increase activity. Optimization of the process using DOE and reaction screening allowed development of an efficient process from the chloroketone to the target epoxide. Epoxide 1 was obtained in high throughput yield with excellent chiral selectivity and purity.
Published in the July 2013 edition of Pharmaceutical Manufacturing magazine.
About the Author
Steve Collier
Ph.D. (former) Director of R&D at Codexis Laboratories
Sign up for our eNewsletters
Get the latest news and updates