Stop me if you’ve heard this one: A Lean TPS guy, a Six Sigma Black Belt and an OpEx Consultant walk into a bar. The bartender looks up and says: “What is this, some kind of Jidoka?” Those familiar with the lexicon of Lean and Six-Sigma, might recognize the term from their Lean 101 primers — in its most Japanese of origins the word means “automation with a human touch.”
But all “jidoking” aside, soon after the Food and Drug Administration (FDA) introduced “Pharmaceutical cGMPs for the 21st Century: A Risk Based Approach,” in 2002, the pharmaceutical industry began to take the concepts of Lean and Six-Sigma more seriously. Sure, several majors implemented programs earlier than that, but over the course of the last 10 years, and in the face of tremendous market and cost/price pressures, the industry has increasingly been adapting to its principles, tentatively embracing the philosophy, and implementing (with varying degrees of understanding and success) the movement’s waste-eliminating, continuously improving principals — applying them to the enterprise in hopes of reducing manufacturing costs and boosting productivity. However, if you come from some other major industrial sector you might be thinking Pharma is a little late to the party — and if you do come from the pharma industry and think that’s true, you’re not alone.
The Japanese, Toyota Production System-led origins of the Lean movement are well established, as are its principles, methodologies and purported benefits. Similarly, Six-Sigma, Motorola’s home-grown statistically based continuous improvement ethic gained its own traction in U.S. industry, especially after Motorola’s Bill Smith coined the term in 1986 and its embrace, strategically, by General Electric’s chairman Jack Welch who, in the ’90s, institutionalized Six Sigma across the company’s global operations. The company unabashedly states that fact on its website: “Today’s competitive environment leaves no room for error. We must delight our customers and relentlessly look for new ways to exceed their expectations. This is why Six-Sigma Quality has become a part of our culture.”
By the turn of the 21st century, a broad swath of manufacturing, from automotive to utilities had, for better or worse, and in one form or another, integrated and institutionalized the combined philosophy’s overall efficiency and quality ethic into operations. While their journey may never be over, few can argue that for thousands of companies, Lean and Six-Sigma initiatives have supported their financial performance and competitive agility while containing production costs and sustaining profitable margins. Industrial output and productivity gains in the ’90s, and resiliency to economic downturns since 2001 point to the general success of the process excellence movement and its contribution to sustaining the economic viability of a broad range of businesses. Unfortunately, corresponding annual productivity increases in pharma, according to one analyst, were “rarely noticeable” and played a minor role as an indicator of operational health.
As mentioned, since approximately 2002 the pharmaceutical industry felt increasingly pressured by a number of new and relatively dramatic external and internal market forces (think patent cliff) and began to face up to its profligate spending sustaining inefficient drug development and manufacturing processes. With the cost of bringing a single blockbuster drug to market reaching some $1.3 billion and, according to Eli Lilly, the success rate of new chemical compounds falling from 12% a decade ago to 8% today, drug manufacturers indeed continue to have a tough fight ahead to remain competitive and sustain commercial success. As late as 2009, there was evidence that pharma’s operational and financial culture was nowhere near ready to embrace the philosophy or make it work to its best advantage. That year, the University of St. Gallen’s Thomas Friedli and his colleague NIPTE director Prabir Basu, announced the results of a 2008 benchmark study that surveyed 160 pharmaceutical manufacturing sites. In an article appearing in Pharmaceutical Manufacturing, Friedli and Basu opened with a startling assertion: “Few managers of pharmaceutical companies see manufacturing as a competitive advantage today. Increasing cost pressures in the industry have led more and more of them to take advantage of global low-cost sources for the production of goods while reducing their own production capacities.” They posited, among other things, that such a strategy, on the surface, made sense, but could ultimately harm the competitiveness of pharma companies in the long term.
THE “KI” TO PROCESS EXCELLENCE
Although it is always dangerous to make sweeping generalizations, veteran pharma industry observers and internal and external experts do ascribe to a general consensus that while Lean Six-Sigma and operational excellence systems are the “Ki” (Japanese for energy or spirit) to produce world-class business outcomes for pharma — the industry still has some work to do before the philosophy and methodologies are truly integrated and institutionalized to produce broad, sustainable gains for the sector. Nigel Smart, principal at Smart Consulting Group and author of the just-released book, Lean Biomanufacturing, says, “For perhaps over a decade now, the pharmaceutical/life sciences industry has been attempting to apply Lean systems to its various process systems. Initially, there were attempts to apply the principles to manufacturing processes in an attempt to mimic the advantages seen in other industrial sectors, such as those in the auto industry. However, if one was to critically prepare a performance scorecard of the implementation of Lean throughout the industry, this analysis would at best give you a normalized score of perhaps 4/10.”
Given the complexities of the global pharmaceutical supply chain in 2013 and the recently clouded performance of the industry in relation to quality excursions, it has become obvious to many that the need to cut operational costs, boost output and increase quality has never been as acute as it is now. But for many in pharma, the journey to Lean is only just now gaining momentum. Smart characterizes it this way: “There is still not uniform acceptance at all levels within organizations across the industry that this is a set of principles that will truly make a difference.”
Smart offers that in many cases, quality management actually benefits from Lean implementation because Lean assures that the appropriate level of resources is applied to each individual process task and that, in many cases, the exercise frees up redundant resources to be used beneficially in other areas. He notes, however, this staccato type of implementation can be exacerbated by emphasizing the award of belt-level projects on specific, often one-time programs instead of adopting a more integrated approach that would truly streamline a company’s operational functionality as well as enhancing its competitive edge.
In some organizations, Smart says, Lean is being employed simply as a resource cutting mechanism and/or an efficiency building tool. “Neither of these address the real value of incorporating Lean into a company’s operating philosophy and culture; frequently viewed by the workforce as another ‘flavor-of-the month’ gimmick that will probably fizzle out within a year as it gives way to the next idea.” So has progress be made? “Absolutely,” says Smart, “but the fault lies in the fact that as an industry we are not universally applying the principles and tools in an integrated holistic fashion that will allow us to fully realize its full potential.”
LEARNING CURVE
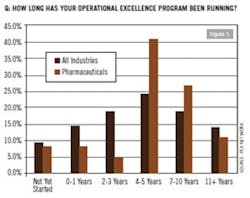
Considering that the rest of the manufacturing industry took at least a decade or more of trial, error and cultural alignment before it started to get more right than wrong from implementing Lean and Six-Sigma initiatives, pharma’s adaptation cycle is proceeding at about the same pace. In late 2011, PEX Network conducted its “Global Benchmarking Study of Trends and Success Factors in Business Process Excellence.” Among the study’s 676 managerial and executive respondents, approximately 6.3% were from the health care sector and another 6.7% from pharmaceutical. According to PEX Network’s findings, slightly more than 40% had operational excellence programs running for the past four to five years (Figure 1). For the most part, the snapshot of data suggests that for pharma, the industry is really just starting to hit its mid-life stride honing the cost-cutting katana of Lean and aligning its methodologies with pharma’s particular culture and operational infrastructure.
So, for pharma, the journey to Lean is beginning to gain momentum and maturity, overcoming, however awkwardly, the status-quo inertia and sacred-cow confrontations that can derail less-than-robust implementations. What’s ironic is the fact that Lean Six-Sigma (even the philosophy has consolidated in terminology to embrace the methodologies of current operational/process excellence programming) is so seemingly right in guiding the pharmaceutical industry as it responds to the regulatory environment and the FDA’s cGMP/Quality by Design (QbD)-based compliance regimes. Lean Six-Sigma methodologies, based on statistical process data and the identifying, understanding and elimination of deviation in process are well-suited to manage quality and provide continuous improvement to myriad pharmaceutical operations. “Quality within all industries is important,” says commentary on Six Sigma Online, Aveta Business Institute’s online Six Sigma certification portal, “but within the pharmaceutical industry it’s essential. Because lives are at stake, quality, when it comes to creating and manufacturing medicines for individuals, is necessary. Because the Six Sigma quality improvement theory boasts less than 3.4 defects per million opportunities, it is worth exploring in any industry, especially this one!”
TEVA’s LEAN QBD APPROACH
In the industry’s generic drug sector, process efficiency is seen to have a higher priority because so much of a given company’s business success is driven by effectively managing product costs.
Earlier this year, Uri Hillel, Head of R&D Quality and Corporate Quality & Compliance outlined Teva’s approach to QbD for Pharmaceutical Manufacturing: “Teva has worked on implementing the FDA guidelines defining QbD guidance and has adopted the QbD philosophy in its development of generic products. For Teva, this means understanding the products, formulations and processes in depth, and submitting appropriate applications to the authorities using a more systematic development approach.”
Hillel’s description of its institutionalization of QbD in support of operational excellence has the unmistakable characteristics of a Lean Six-Sigma-guided system.
“Internal Teva QbD Guidelines,” says Hillel, “were developed. Design of experiments methodology and statistical tools have been successfully implemented at global R&D sites. Research and development is interactively working with Operations, Regulatory and QA on the manufacturing process prior to production of the registration batches, identifying and proposing mitigation to risks at scale up, reviewing together the proposed control strategy, operation ranges and product specifications.”
Friedli’s 2008 study affirmed Teva’s approach noting: “As long as PAT and QbD are managed in isolation, as single pilot projects, we will not see a big impact on the average sigma levels of the manufacturing processes. PAT and QbD have to be integrated into the total plant quality improvement program.”
PEOPLE + INFORMATION
Clearly, when looking across the complexities of a contemporary drug maker’s operational continuum, two elements emerge as critical priorities in pursuit of Lean Six-Sigma-optimized operations: people and information.
It’s generally agreed, especially among pharma’s Lean pioneers, that any programmatic approach to effectively institutionalizing Lean Six-Sigma values into an organization requires that considerable attention be paid to the human element; the role individuals and teams play in supporting and sustaining Lean Six-Sigma operational excellence initiatives. Michael Curran-Hays, a practice leader for Kepner-Tregoe, a management consulting and training company, offered that process excellence in manufacturing is an acceptable vehicle for organizational transformation. “It brings a bias for disciplined action, clarifies the intuitive knowledge gained from experience and puts an organization on the path to accelerated business results. In the quest to attain ‘flow’ with ‘zero’ waste, organizations are falling short on the people management aspect of process excellence implementation.”
Kepner-Tregoe explains that in their experience, the people who are actually responsible for sustaining lean programs can be pushed to the background by cadres of lean gurus. The rank & file’s importance to successful lean journey’s success is often ignored and misunderstood. This, says Kepner-Tregoe, leads to variable and unpredictable process improvements and business results that can’t be sustained. Curran-Hays maintains that when it comes to lean initiatives, focusing on the people piece helps organizations solve the lean puzzle and that the results are translatable consistently across other parts of the organization.
To achieve process excellence, notes Curran-Hays, “We must first understand the landscape, management practices and the performance system that drives project team behavior. To understand the landscape, observe the manufacturing landscape — not as a process excellence expert, but through the eyes of the people who experience waste mitigation first hand as it gets implemented across their organization.” The view, says the consultancy, can be frustrating and confusing. “It is not uncommon to find that the strategic business objectives of an organization are at odds with when and where Lean and waste mitigation are implemented.”
A Kepner-Tregoe white paper offered an example: “A consumer products plant of a large pharmaceutical company decided to transform itself into a ‘Lean Enterprise’ with 37 distinct projects that focused on improving manufacturing flows and fill rates and reducing cycle times. While the projects were implemented, the company was being strategically benchmarked against labor costs in offshore countries.” In this case, says Kepner-Tregoe, a Lean job well done was at cross purposes with the company’s strategic objective to lower labor costs. Results from Lean projects coincided with the closure of the production facility. For people affected by the plant closure, going Lean did not help, it only created disbelief in Lean principles.
According to TayganPoint Consulting Group CEO, Joy Taylor, pharmaceutical companies have recently become more focused on cost reduction and productivity metrics. “For as many clients as we serve, we have seen as many organizational models created to drive operational efficiencies. However, Lean Sigma practices and principles are certainly the most common and are creating unique operational models to fit their culture and infrastructure.” Some, says Taylor, use the centralized model where Lean Sigma is driven by a global Lean Sigma or Productivity group (largely made up of master black belts and black belts) which works mainly on the key cross functional improvement initiatives and provide lean sigma training and support to key initiatives. “The other model is decentralized whereby each function develops its own capabilities to drive Lean Sigma in their functions through functional green belts and black belts. Both models have value, but regardless of the structure, efforts must be strategically aligned to the organizational mission and vision.
In Taylor’s experience, many of her big-brand clients have centralized process excellence groups that provide process improvement and Lean Sigma principles throughout key functions such as the supply chain, support functions, clinical development and aspects of sales and marketing. “But what organizations now require in order to sustain these improvements is a robust enterprise analytics capability; they must be able to track each unit. As such, we see many clients setting up global process owners and governance entities to drive enterprise-wide process management.”
Taylor says her consultancy’s clients have had mixed results from the institutionalization of Lean Sigma and process excellence. “To be exceptional,” says Taylor “requires an all-in philosophy particularly in the supply chain/manufacturing space, which also means you must have access to data. And for some, that is the limitation, hence why ‘big data’ is such a hot button right now. But those who are successful set clear improvement targets and have measurement mechanisms to ensure realization of benefits. For example, having the ability to understand metrics such as OTIF (On Time In Full) or discard metrics can help an organization generate millions in revenue and also save it millions in waste.”
OPEX REQUIRES OPDATA
John P. Helfrich, Sr director, Analytical, Development, Quality and Manufacturing Solutions for Accelrys, finds the mantra in the C-suite for life science companies lately is “operational excellence” from all segments of the supply chain, both internal and external. A key strategic element says Helfrich, to a successful operational excellence effort is capturing and cataloging the experimental and operational data streams as the product transitions from early phase development through pilot and into commercial operations. These data constitute the foundation for true data management transformation to operational wisdom. “As companies initiate Lean Six-Sigma programs and begin the now-popular externalization of processes that previously were performed in-house — from R&D through pilot operations and now into full CMO-based API production and packaging — executive managers are becoming increasingly aware that their information/data management infrastructure requires updating.”
ONWARD
As one surveys the Leaning of Pharma, the journey continues — just like any industry. In spite of the gaps in understanding and implementation, Pharma’s late-coming embrace of Lean Six-Sigma may be a blessing in disguise. The disciplines are well understood, but in Pharma, perhaps not quite as much. The industry’s on a learning curve — but an accelerated one — because it can apply best practices outside the Pharma universe and learn from other’s early missteps. Lean done right can be an amazingly sharp tool to achieve cost effective, efficient and profitable operations. Its disciplines and basis in statistical process control are well aligned with cGMP and QbD theory and practice and support empirically provable compliance when it comes to critical processes. As the Pharma industry responds to the global market’s competitive and financial pressures, its success will be increasingly dependent on operations optimized the Lean Six-Sigma way.
Published in the October 2013 edition of Pharmaceutical Manufacturing magazine
Editor’s note: During my reporting for the cover special feature on the leaning of the pharmaceutical industry,
I had a chance to discuss with Bill Faria, Head of Operational Excellence at EMD Millipore, how Lean principles are being embraced by his organization.
PhM: How do you promote/manage process excellence and waste mitigation across your operational (factory floor, R&D, supply chain) ecosystem?
Faria: “Our Operational Excellence organization is responsible for promoting and managing process excellence and waste mitigation across our operational ecosystem. We have team members in most of our sites and functions responsible for partnering with local leadership teams to understand site and/or functional priorities and to align process improvement efforts to those priority areas. The teams lead and mentor process improvement projects while also teaching a variety of problem solving tools and methodologies utilizing Lean & Six-Sigma principles.”
PhM: Are your operational efficiency initiatives based on Lean Six-Sigma principles? How has your organization institutionalized lean concepts and has it evolved to suit your strategic needs operationally and/or competitively.
Faria: “Yes, our operational efficiency initiatives are based on Lean and Six-Sigma principles. We focus on using the right tools at the right times. We have many examples of Lean concepts implemented in many of our sites (5S, kanbans, visual management, visual work instructions, mistake proofing, etc.). Our program has evolved from initially focusing on cost savings opportunities to a more balanced view of cost, supply and quality opportunities to ensure our process improvements are focused in the right areas. “
PhM: Are there particular aspects of your operational continuum that have received recent scrutiny and the application of Lean principles?
Faria: “One area of opportunity where we are aligning our Operational Excellence team is in Supply Reliability, focused on improving our delivery performance to our customers. Looking at the end-to-end supply chain has helped us identify areas of opportunity to align the teams and improve information flow and process hand-offs.”
PhM: Considering that your organization is actively implementing Lean/Six-Sigma principles to improve and sustain efficient waste-free operations across several fronts, how mature would you say your initiatives and culture are?
Faria: “We have been utilizing Lean/Six Sigma principles for eight years and are still in the early stages of our journey to establish a Continuous Improvement culture. We are past the ‘activity’ phase (initial training, forming teams, starting projects) and are well into the ‘process’ phase (end-to-end process view, cross functional projects, mature training program) of a typical process improvement lifecycle, with an ultimate goal of making it part of the ‘culture’ (the way we naturally solve problems across all levels of the organization).”
PhM: What benefits/returns has your organization realized from the institutionalization of Lean-based or other waste-reducing optimization strategies?
Faria: “We have realized many benefits from our Operational Excellence program over the years. From substantial cost savings to improvements in product and process quality to delivery performance gains to customer satisfaction focused projects and initiatives. We constantly look for ways to utilize the program to improve our business in many different areas.”