Tablets are one of the most popular and convenient solid oral dosage forms used today (IMS Midas Database, 2005). The tablet manufacturing process typically involves mixing active pharmaceutical ingredient (API) powder with a variety of powdered excipients (fillers, diluents, lubricants, binders, glidants, etc.). These powder mixtures are subsequently processed, compressed and mass produced using automated tableting equipment.
TWO MOST COMMON PROBLEMS
Many APIs that are used today are cohesive and have low bulk densities. These properties can pose significant physical challenges when developing a robust manufacturing process. Two of the most common problems encountered in a tableting process when using this type of API include sticking and film formation.
Sticking occurs when particles adhere to the punch face during the compression process. Filming is a slower form of sticking which can occur over time on the punch surface during the tableting process. Film formation and ultimately sticking during compression can lead to problems with tablet thickness, embossment (legibility) and coating. Frequently, film formation and sticking may not be observed until scale up of a tableting process, at which point it may be undesirable or difficult to alter drug formulation parameters.
NOT COST EFFECTIVE EITHER
Tableting processes that are susceptible to film formation and sticking are problematic, inefficient and not cost-effective. In many cases, the compression process must be terminated early or processing times increased (due to the requirement of frequent cleaning and reinstalling to restart the compression process) which may lead to physical properties of the tablet such as tablet thickness and embossing quality to be compromised. Lower tablet yields and long equipment downtimes can substantially increase manufacturing costs and reduce product profit margins.
The goal of the current study was to develop methods to reduce the likelihood of film formation and sticking during compression using a highly cohesive, hydrophobic API in a tableting process. The use of Vortex coolers that reduced and controlled the temperature of tableting equipment during compression substantially reduced film formation and sticking, eliminated equipment downtime and improved tablet yields by 50% without impacting tablet properties/quality.
TABLETING MODS WON’T PREVENT IT
Film formation and sticking can occur when excess moisture or under-lubrication occurs during compression of a tableting mixture. Modification of a tableting process can sometimes reduce or eliminate film formation or sticking during compression without making any drug formulation changes. To test this possibility, modifications including changes to pre-compression force, compression force and tableting turret dwell time/speed were implemented during tableting of a hydrophobic API using a small scale rotary press (Korsch XM 12 Single layer / Bilayer 18 station Tablet Press). Tableting was conducted at turret punch temperatures ranging from 25 degrees Celsius to 34 degrees Celsius.
Varying pre-compression (0.0KN- 2.0KN), compression forces (7.0KN-13KN), turret dwell time (0.10msec-0.30msec) and speed (800-1,200 tab/min) failed to eliminate or reduce film formation and sticking. An inspection of tablets produced from three separate batches revealed decreased tablet thickness and illegible embossments that resulted from film formation and sticking. In fact, each of the three batches had to be prematurely terminated (after 50% of the run) because of product filming and sticking on tableting tooling. In each case, tableting equipment had to be disassembled, cleaned, reassembled and compression restarted. About 1,500 tablets per punch were successfully compressed in each run prior to batch stoppages.
VORTEX COOLERS: LABORATORY SCALE
It is well established that hydrophobic interactions tend to strengthen as the temperature of a process increases. Interestingly, the tableting experiments described above were conducted using turret/punch tip temperatures ranging from 25 C to 34 C. This suggested that reducing the temperature at which compression is performed may help to reduce or eliminate film formation and sticking during tableting of hydrophobic drug mixtures.
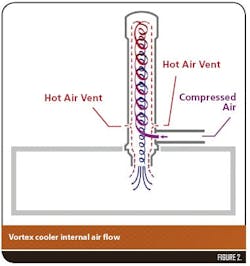
The effect of lower temperatures on tablet compression was first assessed by lowering turret/punch tip temperature of a Korsch XM 12 Single layer/Bilayer 18 station Tablet Press (Figure 1) to 18 C to 20 C by using Vortex coolers (manufacturer details required) (Figures 3 and 4). Turret/punch tip temperatures were reduced to 18 C and maintained between 18 C to 20 C (monitored via an IR thermometer) during the four-hour tableting run.
Cooling of the turret/punch tips during tableting resulted in a 50% increase in tablet yield and a 100% batch completion rate. Moreover, tablet thickness was maintained at 4.56mm - 4.57mm and there were no embossing problems (embossments were fully legible according to Level II AQL testing requirements) or coating issues with tablets manufactured using the vortex-cooled, small-scale tableting machine. Finally, tableting at the lower temperatures eliminated film formation and sticking and allowed batch completion without any downtime.
COMMERCIAL SCALE UP
The successful application of Vortex coolers to a small-scale tableting process prompted additional experiments involving the device and a larger commercial scale tableting machine. Because of the larger size of commercial presses as compared with small scale machines, it was necessary to add an additional vortex cooler to the Stokes D34 (34 station) Tablet Press to cool turret/punch tips to 18 C to 20 C during tableting runs.
Results from these experiments were consistent with those obtained during compression using the vortex-cooled small scale tableting press. Vortex cooling of the commercial press resulted in substantial increases in tablet throughput and batch completion without any equipment downtime. Likewise, tablet thickness and embossments were significantly improved as compared with tablets manufactured using non-vortex cooled tableting presses.
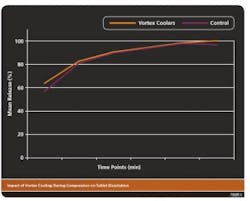
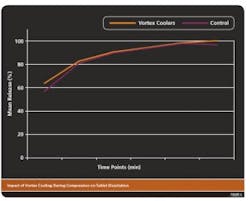
VORTEX COOLING IMPACTSTABLET DISSOLUTIONWhile vortex cooling improved the compression process, it was possible that compression cooling may have altered dissolution properties of the finished tablet. This was assessed by comparing the dissolution rates of tablets manufactured without vortex cooling with those produced using vortex-cooled small scale or commercial tableting presses in standard tablet dissolution tests. Dissolution rates were determined by the amount of API released from the tablet over time in aqueous solutions.The results from these tests (Figure 4) showed that there were no significant differences in the dissolution properties (percent release) of tablets manufactured using either method.Vortex coolers have the potential to be used to improve other manufacturing processes including automated encapsulation, roller compaction and hot melt extrusion in which film formation and sticking may be problems. Additional studies will be necessary to determine a role for vortex cooling in these processes.
References
1. IMS Midas Database, 2005. IMS Health, CT, USA.
2. Zheng, J. (2009). Formulation and analytical development for low-dose oral drug products. Hoboken, N.J.: John Wiley & Sons