The pharmaceutical industry has long relied on stainless steel bioreactors for processing batches of intermediate and final stage products. While these vessels work well in many applications (especially for large batches of 5,000 liters and up), there are many issues better addressed by utilizing single-use bag bioreactors.
This article will discuss the advantages of single-use technologies, and will show how instrumentation challenges can be overcome, particularly for measuring critical process parameters such as pH and dissolved oxygen (DO). These advantages are driving many pharmaceutical manufacturers to retrofit the bioreactor portion of existing processes to single-use.
The allure of single-use
Process and manufacturing industries strive to increase throughput and therefore improve economies of scale. In pharma manufacturing, sometimes this paradigm is superseded by a need for flexibility, along with reducing cleaning and sterilization costs.
Traditional fixed-asset stainless steel technology (SST) is how pharma manufacturing has typically scaled-up operations. Newer single-use technology (SUT) represents an alternate approach, addressing problems with SST and delivering additional benefits.
Even ignoring the initial costs of SST equipment fabrication and installation, operating costs remain quite high since cleaning and sterilizing operations require significant support infrastructure. Equipment and fittings must be cleaned-in-place (CIP) or cleaned-out-of-place (COP) for each batch. CIP/COP consumes large amounts of water and chemicals. The chemicals in question — such as acid, caustic soda and detergents — introduce handling hazards. Much of the water must be of water-for-injection (WFI) quality, which is energy-intensive to produce.
After cleaning, SST equipment must be sterilized-in-place (SIP) with steam, another energy-intensive operation. Occasionally, even after all cleaning operations are properly performed, residue from prior batches remains and can negatively impact the quality of subsequent batches.
Process instrumentation, especially analytical elements, are relatively fragile compared to the equipment, so extra effort is required to protect them during these cleaning methods. Even so, instruments can still be damaged or destroyed, and often require frequent recalibration or replacement.
From a physical standpoint, once SST equipment is installed, there is little or no plant layout flexibility. Small batches and trial runs are frequently not feasible or cost-effective. Looking forward, SST is not well suited for supporting the future wave of small batch and personal medicines.
Systems using SUT elements, such as disposable bioreactor bags, obviate many of the problems experienced with SST equipment and provide other advantages. Chief among these is the fact that single-use bioreactor bags arrive pre-assembled and pre-sterilized from the original equipment manufacturer (OEM). The bags do need to be enclosed in some form of support structure, which is quite minimal compared to traditional construction.
No WFI, CIP, COP or SIP systems are needed to prepare the bags. This provides large energy and emissions savings, especially the relief from needing steam generation for SIP. After a production run is completed, bags are not reused, but are instead sent back to the OEM or elsewhere for recycling or incineration.
With a shelf life of two or more years, single-use bags allow users to easily gauge their stocking needs and deploy new bags quickly. Bags are offered with dual fittings for measurement redundancy and can achieve mixing either with integrated stirrers or by installing them on rocker tables.
Single-use methods give end users more flexibility for scaling up and down, as opposed to being constrained by significant amounts of fixed installed infrastructure. A SUT facility can be built much quicker than a new SST plant, and initial capital costs are much lower.
Traditional fixed-asset stainless-steel equipment requires copious amounts of water, chemicals and energy to clean and sanitize, and instrumentation is often damaged when these processes are performed.
Single-use issues
Single-use methods do introduce challenges, however, especially with the integration of measurement instrumentation in the bioreactor. The sensor element portion of an instrument must contact the product via a fitting in the single-use bag, so this interface must be carefully evaluated. The instrument’s other main component, the transmitter, is not in contact with the process media and thus remains the same for both traditional and single-use processing methods.
Since single-use bioreactor bags are sterilized using gamma radiation, all materials and devices must be able to withstand this procedure. Some sensors use modified designs compared to traditional versions in order to survive this sterilization, so users may have concerns whether these sensors perform as well as traditional devices. This uneasiness was reinforced by original single-use sensors which experienced high drift. However, the newest generation of sensors are improved to be three to five times better than existing SUT sensors, eliminating this area of concern.
One way of integrating and preinstalling single-use pH sensors into bags utilizes a dry storage technique. Unfortunately, some pH sensors of this type can’t be tested until the bag is filled with product and committed to production — far too late to replace a faulty sensor. Another concern is that some preinstalled sensor elements may have a shorter shelf life than the single-use bags, negating the benefit of a long bag shelf life. For measuring electrodes, any exposed glass during storage must be avoided to prevent mechanical contamination.
The physical fittings, also called ports or adapters, used to install sensors into single-use bags have also presented challenges. Fittings must be mechanically designed to meet aseptic requirements. Since the instrument fittings are integral with the bag, selecting the right fitting materials to withstand gamma radiation and maintain aseptic properties is critical. This is verified by conducting extractables and leachables (E&L) testing to ensure the materials will not contaminate batches, even after gamma radiation. Testing is expensive, and compliance can be difficult to achieve for some vendors.
This means that suppliers must choose premium materials with clean natural profiles, typically United States Pharmacopeia (USP) Class VI-tested and free of animal derived ingredients (ADI). Materials must be selected to meet high biocompatibility standards, and USP Class VI materials offer an excellent, well-understood benchmark for all of the different types of polymers used on SUT devices. From an end user standpoint, instruments should connect to SUT bags just as traditional instruments do.
End users want the flexibility of single-use methods, but they want to achieve process control in the same way as with stainless steel methods, with no degradation in performance. These goals can be met by employing the latest measurement technologies.
Focus on pH
Since pH is a critical process parameter for any bioreactor, users need sensors with zero drift and a long shelf life. Traditional pH sensors are high maintenance, especially when installed where CIP or SIP is performed. For these locations, the sensor sometimes must be removed, sterilized separately, then reinstalled for these operations.
For single-use applications, the entire pH sensor must be suitable for a whole production run because it is in direct contact with the process media. Traditional measurement techniques use a glass electrode, and SUT instruments using the same method have similar accuracy.
One significant difference is that traditional sensors have a shelf life of only six to 12 months, far below the shelf life of the SUT bag they would be preinstalled into. Emerson has overcome this problem by developing a unique setup allowing single-use Rosemount pH sensors to be stored retracted and wetted within a chamber containing a stable, proprietary buffer delivering a two-year shelf life.
This configuration allows the sensor to be tested and calibrated while installed in the dry bag before being placed into production, at which time the sensor can be inserted for product contact. The buffer pH can be used as a calibration standard, and a one-point calibration at batch startup results in accuracy within 0.1 pH. Combined with the fact that the sensor has 0.005 pH per day stability, long runtimes up to 20 days are possible after insertion without any required recalibration or maintenance under most conditions. Additionally, one-point standardizations can be performed at any time against an offline sample to reset the sensor drift and continue the run. Thus, the sensors can be used for runs of any length.
Single-use bioreactors offer numerous manufacturing advantages, but the instrumentation requires special attention to achieve performance equivalent to stainless steel systems.
Focus on DO
DO sensing methods are more forgiving than pH for single-use service. The DO sensor itself is the same as for classic SST processing applications and can be reused for multiple batches.
However, for single-use the DO sensor is installed in a fitting with a permeable oxygen membrane, so the sensor has no direct contact with the process media. While this does introduce a small lag time, it isn’t an issue for this type of application where changes in DO occur relatively slowly.
If necessary, the DO sensor can be removed and calibrated on the bench, even during processing.
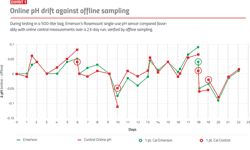
During testing in a 500-liter bag, Emerson’s Rosemount single-use pH sensor compared favorably with online control measurements over a 23-day run, verified by offline sampling.
Single-use sensor applications
New single-use pH and DO sensors and fittings are designed for single-use bioreactors ranging in size from 10 to 4,000 liters. Because the sensors install into 1-inch barb fittings and are gamma irradiated with the bag, start-up is immediate.
These sensors can be employed in any application where a bag is used, not necessarily confined to only upstream fermentation and perfusion applications. With a large 2 to 12 pH sensing range, the pH sensor can also be used for in-bag applications involving viral inactivation, tangential flow filtration, and others.
In one test of the early pH prototypes in a 500-liter bag, Emerson’s Rosemount sensor maintained stability much longer than the other online control sensor. Exhibit 1 shows the difference between the single-use online pH sensor and offline sampling measurements taken over this 23-day run. For this test, users triggered a one-point standardization if the online pH sensor deviated more than 0.05 pH from the offline measurement for two samples, or if the online sensor was ever more than 0.1 pH off from an offline measurement. The single-use sensor compared favorably to the control sensor, requiring only a single one-point standardization versus the four required for the control.
Finding the right answer
In many processing and manufacturing sectors, scaling up operations with large production equipment installations is the path to efficiency. Within the pharmaceutical industry, however, SST bioreactor installs are not always the right answer. Adopting SUT technologies like bioreactor bags minimizes the need for cleaning, sterilization and the associated expenses for WFI, chemicals, energy and supporting infrastructure. Single-use also offers newfound flexibility to scale operations up and down.
Since many of these processes are automated and all must be monitored, there remains a need for pH and DO instrumentation, which must use installation and calibration methods suitable for this demanding service. Updated instrumentation is now available to maintain the performance and familiar form factors of traditional instruments, but with enhancements to deliver long shelf life while meeting single-use sterility requirements.