Harmonizing technology across the pharma lab and production floor
This and other measurement issues can be resolved by standardizing the technology used throughout the product life cycle, from process development to pilot to full production. And with modern developments in electronic sensors, the same instrumentation types can be used in the lab where stringent tests must be conducted to ensure quality, and in production where fast and accurate measurements must be made to keep operations on schedule.
By standardizing and using the right instrumentation throughout a product life cycle, measurement discrepancies and subsequent production and business procedural disruptions can be reduced. This article will examine some of the problems encountered in the pharma industry when using different technologies in the lab versus in production, and discuss how modern digital electronic instruments for liquid analysis can solve these issues.
The need for reliability and consistency
As process analytical technology (PAT) becomes more widely implemented throughout facilities, there is a growing need for proper instrument lifecycle management in the lab and production environments. By using digital sensor technology, the best practices used for instrument life cycle management in lab environments can be extended to instruments used in pilot plants and production environments.
Process analytical technology is driven by the U.S. Food and Drug Administration to design, analyze and control pharmaceutical manufacturing processes through the measurement of the critical process parameters affecting critical quality attributes. PAT aims to improve production by defining the critical process parameters, monitoring these variables in real time, and using the measurements to ensure quality.
The goal of PAT is to develop processes that consistently generate higher quality products, while improving efficiency through increased automation, reduction in cycle times, and uninterrupted batch and continuous processing. Such processes minimize rejection of products, thus improving efficiency.
Uninterrupted processing also improves energy consumption, material usage and capacity, generating significant benefits by improving the coordination of instrument sensor management among lab, research and development and production environments.
PAT is being implemented across the pharmaceutical industry, with a heavy reliance placed on the sensors used to monitor variables deemed critical to product quality in the production process. This need for advanced sensors in production environments does not take away from the need for lab-based sensors but instead drives the need for a common best-practices approach for both lab and production sensors.
With digital sensor technology and lab-based sensor life cycle management tools, the same sensors in the lab and production can be managed using the same approach.
Modern solutions for liquid analysis
Advanced instruments include self-diagnostic features, providing users with device health and calibration information, in addition to primary and secondary process variables. Paired with waterproof, inductive electronic technology, these types of smart instruments are revolutionizing liquid analysis for lab and production settings.
Digitally-enhanced sensors are useful for measuring pH, conductivity, dissolved oxygen and other properties. Newer pH probes use specially designed glass membranes to enhance performance, and reference gels that improve measurement accuracy and decrease sensor degradation rates in harsh operating conditions, such as extreme temperatures, clean-in-place, steam-in-place and autoclave cycles. These devices convert a measured process value to a digital signal, and then transfer it inductively to a transmitter, with the sensor/transmitter pair constituting an instrument.
This technology eliminates problems associated with exposed metal and moisture effects, such as corrosion, ground loops, electrical interference, and more. The flexibility of its application in both mounted and handheld probes makes these sensors ideal for permanent or spot measurements in virtually any location.
New electronic sensor technology can store calibration information directly on each device, making it available for consumption alongside sensor diagnostic and process data. The technology provides detailed process review and trend identification, precise data management, and a basis for predictive maintenance and industrial internet of things services.
Non-contact digital data transmission eliminates the effects of moisture and corrosion, and galvanic isolation ensures interference-free measurement and electromagnetic safety. Many of the transmitters communicate via multiple digital fieldbus protocols for simple integration with existing equipment in the lab, and with control systems on the plant floor.
Applications
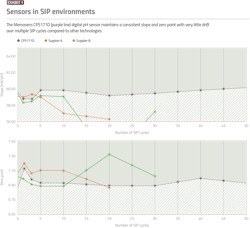
Processors can improve measurement reliability and reproducibility with electronic sensor technology because inductive signal transmission is not prone to the same breakdown and drift associated with metal-to-metal connections. This makes these sensors especially suitable in SIP environments, where temperatures regularly reach 248 F or higher, and moisture is a concern. While conductive sensor/transmitter instruments may succumb to these harsh conditions over a short period, modern electronic instruments can last many times longer through numerous SIP cycles (Exhibit 1).
Additionally, real-time diagnostics and health data make it easier to manage these sensors, facilitating proactive cleaning and calibration schedules because diagnostics can preemptively alert staff when service is required. Pre-calibrated sensors with inductive connectors make onsite exchange easy, enabling plug-and-play handling to increase process uptime.
An example where these benefits are apparent can be found in a four-pole conductivity sensor, using four conductors to measure conductivity. This design allows the sensor to operate over a broader measurement range than traditional two-pole conductivity sensors, while providing increased diagnostic data. The digital sensor head digitizes measurement signals and performs several internal instrument health checks, providing a host of performance and diagnostic data, in addition to process information.
With digital sensors and transmitters in sync throughout a facility, data can be easily communicated among lab, process, a host control system, and on up to the enterprise level. Data is no longer limited to the primary process variable, but now includes secondary process variables, sensor health, sensor performance characteristics, calibration information, and real-time diagnostics. All this information can be used to improve processes, optimize an instrument’s performance, extend its life, and maximize the productivity of operations and maintenance personnel.
These digital-based devices and systems are being further transformed with the industrial internet of things, taking the industry to places unimaginable at a time in the not-too-distant past.
The transformation of pH optimization
Historically, pH measurement systems — and their associated sensors — were applied specifically for use in each unique environment. pH measurement systems used in the lab were managed in the lab, while those used in pilot plants and on production lines were governed separately.
For example, an analog-based pH measurement system must be managed at its point of use with the sensor, cable, and transmitter calibrated together (Figure 4).
Calibration is easier to accomplish and maintain in the lab because the sensor, cable and transmitter are all located next to each other. The lab system is typically validated and calibrated under controlled conditions, but this is not typically true of production systems.
To calibrate an analog sensor installed in a production process, a technician must bring all the necessary cleaning and calibration materials to the measurement location. The sensor is extracted from the process, cleaned, and calibrated, but the measurement is lost throughout this entire procedure.
Unlike traditional analog sensors, digital pH sensors (Figure 5) convert an analog mV measurement to a pH value using a calibration embedded in the sensor head. Digital sensors also record and store several performance data points, enhancing individual sensor life cycle management efficiency. With digital sensors, the same sensors can be used in the lab, pilot plant, and production — all managed using common software tools.
Handheld systems for verification
In addition to reliability enhancements available with electronic digital sensor technology installed at fixed points in the lab and production, instrumentation manufacturers are now incorporating the technology into handheld systems for verification spot checks. This provides processors with the ability to ensure compliance at the most critical control points using consistent sensor technology while providing troubleshooting assistance when measurement issues are identified or suspected.
These handheld systems incorporate many of the same digital characteristics as their fixed-point siblings, including sensor health and diagnostic data, multiple real-time process variables, and time-stamped, tamper-proof calibration and traceability data. This information can be easily accessed using Bluetooth communication and mobile app interfaces for connectivity with a variety of consumer- or industrial-grade mobile devices — such as smartphones and tablets — easing documentation and sensor configuration procedures.
Enhancing operations in the lab and process
Labs serve a critical role in product life cycle development, and provide quality checks on production process parameters, necessitating instrumentation consistency. Coupled with the digital advancements of smart sensors, this makes instruments with electronic sensors often the best choice for liquid analysis in the lab and production.
Processors can increase development, production, and business efficiency by standardizing with this type of instrument technology for improved measurement reliability, longer instrument life, preemptive maintenance alerts, holistic process and diagnostic data, and documentation integrity. Additionally, standardizing on one instrumentation type eases the process of scaling from R&D to process development to pilot plant, and finally to production, by consolidating hardware and life cycle management tools.
About the Author
Thomas Chirdo
National Product Marketing Manager, Liquid Analysis, Endress+Hauser
Bethany Silva
Life Science Industry Manager, Endress+Hauser
Bethany Silva is the Life Sciences Industry Manager for Endress+Hauser, a globally operating process and laboratory instrumentation and automation supplier. In this capacity, she is responsible for the strategic direction, vision and growth of the Life Sciences Industry in the U.S. Bethany has worked with several life science companies with positions in research and development, process development, marketing and technical sales. She brings more than 20 years of experience in sales and marketing to the role, 13 years dedicated to life sciences.