Buffers are essential in all stages of the production of biological drugs, and therefore important for every manufacturer. Managing the logistics and large number of components involved with buffer manufacturing and storage requires extensive amounts of time and labour, as well as facility space. This has led to an increase in the number of manufacturers seeking alternative options to in-house preparation.
The decision whether or not to outsource buffer preparation is not clear-cut but provides an opportunity to balance risk with reward. Irrespective of the size of the organization and in-house capacity, outsourcing can:
- Help increase process efficiencies
- Reduce risk by simplifying and standardising workflows
- Help decrease operating cost, and improve quality, compliance and productivity
- Free up valuable skilled staff, as well as space, for value-add tasks
In general, outsourcing is an increasing trend that has been emerging in the industry over the last six years, and buffer preparation is an obvious candidate for many organisations. This is particularly true for downstream processes, where the volume and number of buffers required for multiple purification steps is significant and complex.
Even when batch sizes are small (e.g. for research or cell and gene therapy applications), there are significant benefits to be gained from outsourcing buffer supply. It can enable organisations to stay capital-lean and maximise their limited resources most effectively. It also lays the groundwork for easy scale-up and provides an assurance of supply.
The clear opportunities offered by outsourcing are accompanied by concerns such as a sense of loss of control, a risk that quality may deviate from what is expected or required, or a potential lack of integration with your own processes. However, these issues can be reduced or eliminated with thoughtful planning and collaboration.
When outsourcing, there is no one-size-fits-all but a continuum of available options, and ways to minimize the risks. When this decision is approached in the right way, there is huge potential to make operations more economical and efficient. There are also a number of advantages that are afforded by supplier programs (such as safety stocks and excess inventory), which should be factored into the decision-making process. In addition, manufacturers can benefit from enhanced quality control and efficiencies of scale that may be otherwise difficult to achieve.Benefits to gain from outsourcing
Reduce testing, ensure quality
When deciding to outsource, it should be kept in mind that there is an extensive amount of work that can be done by the supplier. Responsibility for procurement and QC testing of salts, liquid preparation, filtration, quarantine and finished good testing, as well as all the documentation, procedures, validation and training that accompany these, are all removed when outsourcing. Simply managing all aspects of the buffer production process and scheduling these to fit ‘just-in-time’ product manufacturing processes are tangible benefits that can also be gained.
Buffer performance does not need to be compromised. Selecting an outsource partner with a robust quality management system and employing high-functioning process and continuous improvement approaches will ensure the production of the highest quality buffers. Product consistency can be enhanced by preparing buffers in the larger batch sizes that are achievable when outsourcing, compared with the multiple, smaller batches that are often produced when buffer is prepared in-house. This coupled with in-depth knowledge of liquid stability can potentially enhance buffer performance.
Maximize facility footprint
Buffers are the largest component of downstream processing by volume and, as such, storage can be a challenge for many manufacturers. The need to store 25,000L of ready-made buffer, for example, creates a large amount of facility dead space. Even with appropriate temperature warehousing, once prepared, every buffer has a shelf life after which it becomes unviable. Preparing and storing buffer in-house risks wastage and loss of investment if the full amount of the buffer cannot be used in time.
Buffer preparation and storage for a standard monoclonal antibody can require up to 30 percent of a facility’s valuable floor space, which could be otherwise used to expand manufacturing capacity. A simple solution to reclaim some or all this footprint is to outsource buffer preparation entirely, eliminating the need for mixing tanks and their maintenance, with the added benefit that capital and operating costs are reduced as a result.
Utilising an outsource partner’s cGMP warehousing and taking advantage of just-in-time delivery, so that only the right amount of buffer is delivered exactly when it is needed, significantly reduces on-site storage needs. Removing these constraints opens up the opportunity to repurpose facility usage and support growth.
Benefit from additional support and insight
Accessing a partner’s depth of experience and understanding can be invaluable. For example, a chemical capability team can highlight any concerns about solubility limits or other formulation challenges. They can also provide critical insights on appropriate packaging and help avoid mechanical failures or exposure to any unwanted extractables or leachables.
Additionally, some formulations are more complex than others. This can cause challenges when it comes to preparation and storage. The need to handle caustic or toxic chemicals, or special production conditions such as cold processing to maintain stability, can very quickly drive an escalation in production costs.
By offering a technical sounding board, a partner can make recommendations that could improve your formulation, pre-empt issues and help ensure ready integration with your processes.
Drive operational excellence and continuous improvement
Outsourcing buffer preparation can also provide the opportunity to implement new technologies and approaches to stay at the forefront of the industry. The benefits from outsourcing buffer preparation can play an important part of driving efficiency and continuous improvement efforts for an organisation.
Do the economics make sense?
When you’re prioritizing the assignment of finite capital investment funds, outsourcing non-core functions is an obvious option. For outsourcing large volume liquids, specifically buffers, basing this important decision on data is vital, since there is often more than one option that can deliver measurable benefits.
Understanding your current costs is a key first step, and an economic modeling tool is a good way to define these by quantifying the trade-offs and helping identify the potential benefits that can be achieved. By inputting data unique to your facility (such as detailed utility spend, labour costs, equipment, building cost, and buffer ingredients) it is possible to calculate a breakdown of annual expenses related to buffer production, and to determine a cost per litre. The analysis will facilitate informed outsourcing decision making.
As well as directly comparing overall costs, the cost-efficiency of individual steps should also be considered. For example, buying pre-weighed components can remove the time and effort needed to dispense and weigh the required raw materials, as well as reduce any requirements for special handling capabilities.
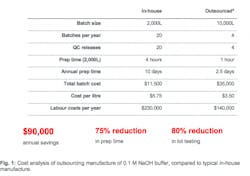
Using the common buffer formulation of 0.1 M sodium hydroxide (NaOH) as an example, Fig. 1 shows how attractive the economics of outsourcing can be, simply based on the larger batch sizes achievable through the use of an outsource partner. The five-fold increase in lot size compared to the typical lot size in-house translates into significant annual savings, as well as reductions in prep time, lot testing, and per-litre cost.
Even for organizations who produce large volumes in-house, facility redesign and new facility future-proofing can often tip the balance in favour of an outsource option. A new facility, or switching to single-use, can be an excellent opportunity to increase productivity per square meter by outsourcing non-core elements like buffer production.
Can you afford not to outsource?
Once you’ve made the decision to outsource, the key to making it a success is to choose an outsource partner that can accommodate your needs, whether you’re looking for flexibility of buffer packaging or format, proximity to your facility, manufacture in a cGMP environment, or simple cost savings.
Getting it right from the start is vital; invest time upfront to ensure that priorities are clear and the production needs can be met. Evaluating options, such as powder or liquid formats and types of containers, can be daunting; however, the resulting product should be easy to use and fit right in to your existing process.
Working closely together will result in the identification of a tailored solution — it’s about finding a solution that’s fit-for-purpose and cost-effective. With the compelling economics, outsourcing is implemented by more organizations every year. And ultimately, it could be more of a risk to continue doing business as usual, rather than to outsource.