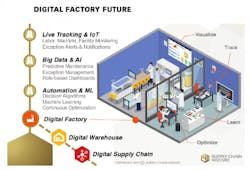
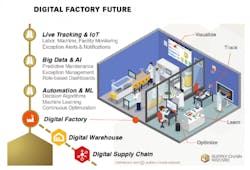
A great many contradictory predictions about the future of pharma manufacturing have been put out into the universe. Some forecasting focuses on looming serialization compliance requirements and their potentially negative impact on packaging and supply chain operations. Others highlight the bright side and focus on the potential impact of emerging technologies, such as the Internet of Things (IoT), artificial intelligence (AI), machine learning (ML) and blockchain.
Of course, the future will vary company to company. Much will depend on the operational strategies chosen — whether these decisions be conscious or subconscious.
Digital vs. Traditional Factory
Let’s explore the differing results that may ensue if companies take a digital versus traditional approach to problem solving for various business challenges. We’ll call these approaches “Digital Factory” and “Traditional Factory,” and imagine five scenarios played out five years into the future, in 2023.
I will highlight the vastly improved results that I predict for those who choose a digital approach. It is my firm belief that with the accelerated rate of change in digital technologies, and the enablement of track and trace infrastructure, a “Digital Factory” approach will result in massive improvements in data availability, visibility and decision-making mechanisms.
Scenario 1: Bidding on a New Business Opportunity
Case Study: A new RFP is received from a major customer looking to select a new CMO partner to produce a high-volume product portfolio, which could increase current demand volume by 30 percent.
Traditional Factory: Management assumes it can fulfill demand without a realistic capacity or cost analysis. Previous RFP submissions are leveraged to benchmark the best price quote, and a final offer is submitted with adjustments to these benchmarks based on management’s subjective comments on the negotiation power of the customer and his expectations. If the business is won, the management team struggles to meet demand at all costs.
Digital Factory: Product and volume mix of new business is used in the existing digital capacity model to precisely know the capability to supply, together with implications on internal costs such as additional staff needs, shift restructuring, batch-size driven OEE assumptions, etc. After a realistic cost/supply-side analysis is performed, a reasonable price proposal is estimated based on target gross/net margin assumptions. Multiple scenario analysis is conducted on various volume vs. price points to determine the right negotiation strategy. If the business is won, the management team knows exactly what to do to adjust operational parameters.Scenario 2: Handling a Crisis — Facing Potential Loss of a Major Customer
Case Study: CMO’s largest customer is approached by an alternative supplier and must make a major decision regarding whether or not to transfer 50 percent of its business.
Traditional Factory: Management tries to keep the customer’s volume by offering price discounts and other incentives. If the loss is final, management takes reactive actions to introduce severe “across-the-board” cost-cutting to direct and indirect labor and spending to manage this existential threat.
Digital Factory: Using its digital toolkit, management analyzes various “what if?” scenarios of lost volume/revenue in real-time, and quickly ascertains the most suitable strategic operational response. Sales and Operations Planning executives utilize data analytics to oversee the decision making process, calculating outcomes to key metrics such as profitability, and determining the appropriate set of cost-cutting initiatives based on root-cause-analysis and the estimated extent of impact to various business segments.
Scenario 3: Capital Expenditure and Investment
Decisions for Capacity Expansion
Case Study: After two years of steady growth in business volume, management decides to expand capacity by building a new facility or expanding current facility capacity.
Traditional Factory: Management decides to buy additional equipment immediately, based on the expectation that the volume growth will sustain and require new capacity in the long term. If the capital expenditure (CAPEX) is limited, management will try to maximize overtime labor, creating a high-cost operational setting to meet the capacity needs.
Digital Factory: Management analyzes the need to add new equipment (or a new site) under various business volume and efficiency scenarios, maximizing the throughput on existing equipment under various shift schedules, and undertakes new CAPEX investment only if truly necessary. Digital analyses highlight bottlenecks on capacity; based on this data, investmest is made in solutions to target these bottlenecks, minimizing capital expenditures while maximizing capacity for a balanced cost/capacity framework.
Scenario 4: Demand/Supply Balancing
Using Scheduling
Case Study: High volatility of customer orders requires significant over-time labor cost, so management decides to look at staffing levels and contingent labor strategy.
Traditional Factory: Management hires more labor on a contingent basis and/or asks current staff to work overtime during third shift or weekend shifts. Both scenarios create a high-cost structure, and while the overall utilization stays low, costs go up to manage volatility of customer orders.
Digital Factory: Management analyzes changes in order of patterns via digital solutions and order/schedule history, and maximizes the utilization of existing staff, while minimizing overtime hours to meet the demand. If customer orders are becoming more volatile, the right balance of staff levels and contingent labor, as well as overtime capacity, is determined based on various scheduling and order scenarios. Digital scheduling solutions help run various simulations to quickly determine the ideal operational parameters.
Scenario 5: Managing a Quality Event or Customer Complaint
Case Study: A complaint is filed from a wholesale customer about a damaged label and a missing unit on a controlled substance product.
Traditional Factory: Management starts an investigation based on this deviation, and makes macro-level decisions to put a hold on all processes that may have led to this adverse event. Due to a slow data collection process across disparate systems, the process can take weeks or months before a root cause can be identified and mitigated.
Digital Factory: Management utilizes all available digital tools to expediently investigate the full history of this specific product down to serial number granularity. All related processes — from manufacturing and packaging to warehousing and shipment — are consolidated within minutes to analyze for non-compliance to business and quality rules and procedures. Any deviations from these standards are analyzed at the serial number and batch level to isolate the root cause. Corrective and preventive actions (CAPAs) can then be implemented based on data-driven insights, further strengthening the processes attributing to this deviation.
A Digital Journey
To achieve long-term success with the digital transformation journey, pharma executives would benefit from a three-phase approach:
Phase 1: Track and Trace/Serialization Compliance and Aftermath
Manufacturers should consider going beyond bare minimum compliance with the U.S. Drug Supply Chain Security Act (DSCSA) and the EU’s Falsified Medicines Directive (FMD) of serializing each unit of pharmaceutical prescription drug. It would benefit them to look at the aftermath of packaging operations and supply chain operations efficiency losses, exceptions and related process changes.
Some leading contract manufacturers have already started realizing an increased level of exceptions and operational challenges as they ramp up serialized products, and may want to consider baselining existing “as-is” operations before fully ramping up to a serialized “to-be” operational mode. In other words, there is heightened value in managing Overall Equipment Effectiveness (OEE) metrics very closely. For CMO/CPO businesses, every percentage loss or gain counts, and it would be a competitive advantage to manage operations in a sustainable way.
Phase 2: Digital Transformation
The transformation of factory operations could be simplified into two broad categories. One is the digitalization of any paper-based processes, such as the management of logbooks and batch records, and the leveraging of digital solutions to eliminate cumbersome manual efforts and lack of visibility on any paper archives.
The other is the digitalization of any “spreadsheet-based” tracking sheets, models and processes, such as capacity planning, detailed scheduling and resource management. Handled separately across many versions of spreadsheets, and managed by multiple stakeholders independently, these processes do not generate the required level of agility, precision and robustness. Additionally, many of these spreadsheet models are often limited by the analytical capabilities of the owner/builder, and are open to human error.
This web of manual processes and spreadsheet models slows down the factory decision making process, making businesses less competitive in an increasingly dynamic marketplace. Creating a roadmap to digitize these recordkeeping/data solutions, as well as dashboard and decision solutions over time, can unlock significant value.
Phase 3: Advanced Analytics and Decision Automation
Once the foundational systems and processes are in place, it’s time to move into more sophisticated technologies, such as IoT-based wireless sensors for continuous facility monitoring that can enable business-rule-driven alerts and notifications. Additionally, leveraging the abundance of data and using artificial intelligence to predict downtime (aka preventative maintenance) or detect anomalies in factory processes can offer significantly more value beyond the traditional digital solutions.
Finally, when the digital flow of data and digitized processes are in place, some decision-making processes can be fully automated for minimal human intervention, saving time and adding precision to supply/demand balancing. Additionally, more advanced algorithms using machine learning capabilities can bring hidden insights to the surface, helping executives better understand operational weak spots so they can invest in these bottlenecks to strengthen their operational robustness.
Which Choice Will you Make?
Over the next five years, pharma companies that embrace a digital factory approach are likely to enjoy much greater success than those retaining a traditional approach. Taking this assessment one step further, pharma companies not only should consider digital factory strategies, but also start implementing initial pilot programs to explore the value they can bring to operations. Very often, early proof pilot projects help create momentum and achieve “quick wins” that help fuel future transformation efforts and, possibly, unlock funds to reinvest in long-term business needs.