The life sciences industries in general and pharmaceutical manufacturing in particular are subject to a wide variety of regulations covering production techniques and equipment design. This isn’t exactly news to anyone in the business, but stop for a moment and consider what it means on a practical basis, and how these constraints affect day-to-day activities.
For a long time, the U.S. Food and Drug Administration (FDA) has regulated pharma manufacturing facilities. Its collection of written regulations provides a wide variety of thou-shalts and thou-shalt-nots, but they often lack details on how these regulations need to be applied. As a result, life sciences companies will look to sanitary standards, such as 3-A, to provide guidelines of equipment configurations.
Let’s look at a specific example of how this kind of regulation affects behavior related to process instrumentation. Say an engineer wants to add a pressure transmitter to a bioreactor. There are dozens of units on the market from various suppliers suitable for the application based on the needed range and precision. But, probably more than 95 percent of the options cannot be used because they do not meet requirements imposed by the regulations.
Those with threaded connections or a recessed diaphragm need not apply. Ditto for devices with too coarse a surface finish, or the wrong shape. When the specifications necessary to satisfy the regulatory requirements are applied first, the number of possible products drops to a handful, and often less, depending on the application.
Inviting Life Sciences to the Smart Table
To someone who has had to make this kind of selection on a daily basis, such restrictions are simply a fact of life. But there are unfortunate side effects which are often overlooked. The instrumentation industry has changed enormously in the last 10 to 20 years. The sophistication of smart devices has brought new diagnostic capabilities and communication options to users in a wide range of applications, but not always life sciences.
For many years, regulatory requirements have kept many pharmaceutical producers stuck with basic instrumentation. Regardless of all the advances made across the board with process instruments, when the first selection criterion is hygienic configuration and compliance, none of the others matter. Fortunately, the picture is changing as more smart instrumentation is becoming available using hygienic designs, offering improved performance in many areas.
What Makes a Smart Instrument Smart?
Returning to the earlier example, a basic electronic pressure instrument delivers an analog signal, most likely 4-20 mA. Some sort of digital panel meter or larger automation device, such as a programmable logic controller (PLC), will convert the analog signal to the appropriate engineering units. That’s all the instrument does: It provides one process variable, which might be all that is necessary for the application.
A smart pressure transmitter can provide the basic process variable while simultaneously sending additional information via a digital signal superimposed over the analog value using the HART protocol. Alternatively, a smart instrument may use some other digital communications protocol, such as Foundation Fieldbus, but this assumes an existing fieldbus installation, whereas HART can be easily applied in retrofit applications.
A smart instrument can send additional process variables, such as the temperature it is sensing, and it can also monitor its own condition and performance. If something is going wrong with the circuitry used to process the data, it can send a warning of an impending failure. It can retain its own calibration history, and even send a signal if it is approaching the end of a calibration interval. Transmitters with WirelessHART communications can do the same thing, all without the need for cabling.
Typical Applications
Picture a large bioreactor, a 5,000-gallon vertical tank with various fittings, a heating/cooling jacket and an agitator. How might this unit be outfitted with various types of smart instrumentation?
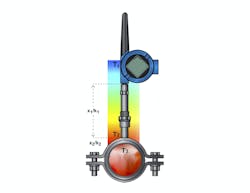
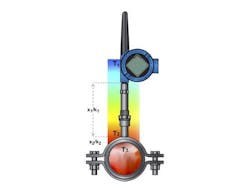
Measuring level—To measure liquid level in the reactor using a conventional approach, a differential pressure (DP) transmitter can be used. A diaphragm seal and capillary is connected to the low side of the transmitter at the bottom of the tank and runs to the headspace at the top so the level reading can be corrected when the reactor is pressurized.
A more sophisticated approach uses two smart pressure transmitters, one mounted at the top and one at the bottom (Figure 1). Rather than an impulse line between the two, they are connected and synchronized electronically, so the headspace pressure reading does not have to be sent via a physical connection.
Using two smart pressure transmitters in an electronic remote sensor configuration can provide more data about what’s happening in a reactor, beyond simple DP level measurement.
The primary transmitter at the bottom of the tank makes a calculation using the pressure sent by the secondary transmitter to correct the level reading. It then sends the compensated reading to the automation system as a single value. This solves the basic level reading problem, but it does more. The secondary transmitter at the top can send the headspace pressure value, indicating overall reactor pressure. This reading may be very useful during a fermentation process when gas is being produced.
It can also detect changes in the specific gravity of the contents. If some nutrient in the solution is being consumed and turned into something else, the density will usually change. This type of setup can be used to detect when such changes stop, signaling a new phase in the reaction.
Measuring temperature—The bioreactor has thermowells installed at various positions which can accept thermocouples or resistance thermometers (RTDs) connected to a temperature I/O card for the automation system. These monitor the reaction, and also verify appropriate temperatures have been reached for cleaning and sterilizing operations.
Basic temperature sensors can perform their normal task and be quite accurate if everything is intact and functioning correctly. Problems often develop if the sensor begins to deteriorate, which might not be immediately recognizable.
Smart temperature transmitters can use a variety of approaches to verify sensor reliability and accuracy. Most configurations use a transmitter at the sensor to process the signal before sending it to the automation system. This provides several advantages.
Single transmitters can actually be outfitted with multiple sensors operating in parallel. The transmitter compares the readings to make sure there is no conflict, which might suggest a developing problem. If one sensor fails, the other serves as a backup while the transmitter sends a message to the automation system warning of the problem. Other approaches might involve checking the condition of a thermocouple by taking resistance readings across the sensing junction to detect any deterioration. These functions can be easily programmed into the transmitter.
Given the importance of temperature in most pharmaceutical manufacturing processes, this helps provide the highest accuracy and reliability.
Level switches—One very useful process diagnostic tool is a vibrating-fork level switch. These are usually placed in the side or on top of a vessel to determine if liquid is present or not at that point, but they have more uses. A vibrating-fork level switch has two small paddles extending into the reactor space. These are connected to a piezo-electric driver that causes them to vibrate at a specific frequency.
If something interferes with the vibration, such as immersing the paddles in liquid, the frequency will be disrupted, which the unit can detect and characterize. This means it can determine if it is in open air, immersed in liquid or immersed in foam. It can thus differentiate between liquids of different densities. Using smart features like delayed switching can even ensure false signals aren’t sent, like from turbulent levels or spray balls, while true high-level alarms are detected
These are just a few examples. There are many types of smart instruments now available in hygienic configurations suitable for the four main process variables: flow, temperature, level and pressure. There are analytical instruments as well for measuring conductivity, oxidation-reduction potential (ORP) and so forth.
Of course, not every measuring approach is suited to hygienic configurations. The necessity of avoiding crevices and providing smooth interiors makes some flow measuring techniques impractical, but there are usually alternatives. While some DP flow technologies are difficult to adapt, Coriolis flow meters are ideal and can provide an enormous amount of information about their operation, and the characteristics of the fluid flows they are measuring.
Smart Instruments in Validated Processes
One question that might arise in this discussion is the ability to add a smart instrument to an existing application which has already been validated. Naturally, each situation is different and every company will have to make these decisions individually, but if a hygienic-design smart instrument has the same capabilities as the basic instrument it is replacing, it should be able to fulfill the requirements. This is because the additional functionality of the smart instrument normally goes beyond the original requirements and does not interfere with the basic process variable measurement.
The additional information, such as secondary variables and diagnostic data, has to be captured separately. Some automation system platforms have the capability to decode HART data natively. All that is necessary to use it is adding the right functionality to the control code. Others may need an external device such as a HART modem to lift the information off the conventional signal wires. WirelessHART instruments deliver data via a gateway hardwired to the automation system.
Diagnostic data usually has to be coupled with an appropriate asset management system designed to watch hardware condition continuously. Using historical data, these systems can identify when something has changed, usually before an outage interrupts production. Maintenance departments can receive notifications and take appropriate action.
Non-Invasive Measurements
As mentioned earlier, many smart instruments can report secondary and tertiary variables, such as capturing a temperature reading from a pressure transmitter. These free readings can avoid the need for installing another instrument if used under the correct circumstances. But there are a few types of additional instruments that can be added to a process without any modification to the equipment or unit operation.
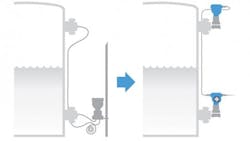
One typical example is an external temperature sensor. Smart instrument technologies have been applied to surface-mounted RTDs designed to measure the temperature of a process fluid directly through the pipe wall (Figure 2). The sensor is held in firm contact with the metal surface to ensure reliable heat flow, and the transmitter performs a series of calculations based on the heat transmission characteristics of the pipe material and wall thickness, plus compensation for ambient conditions.
A temperature reading through a pipe wall can be very accurate if appropriate calculations are made to compensate for heat transfer characteristics and ambient conditions.
Using all these factors, it can create an accurate process temperature reading for the fluid without any process penetration. Everything is external so there is no contact with the product. The assembly can be mounted to pipes of any size greater than one-half inch, accompanied by insulation. This approach can provide data via a conventional wired or WirelessHART transmitter. Such an instrument can eliminate the need to add challenging intrusive temperate points, or moved as needed during validation or troubleshooting procedures.
Adapting to Life Sciences
These new possibilities emerged because instrumentation providers have responded to the requests of life sciences companies. This has involved extensive modification of the components to satisfy requirements for material choice, surface finish, internal geometry, connections, clean-in-place (CIP) and sterilization-in-place (SIP) capability and other specialized demands. The electronic parts are usually the same as their conventional counterparts, so reliability is assured.
Users working in pharmaceutical manufacturing may need to reexamine their underlying assumptions about instrumentation, and how they might be able to use new technologies. There are many products on the market designed specifically for these applications, so it is no longer necessary to live with reduced capabilities to accommodate regulatory requirements.
[javascriptSnippet]