The biopharma sector has seen incredible growth in recent decades, with growth the major long-term trend. Total annual revenue has increased from about $4.4 billion in 1990 to now about $275 billion, an increase of 6,250 percent, with biopharmaceuticals now more than 25 percent of the total pharmaceutical market.
For over two decades, the biopharmaceutical manufacturing industry has created a body of industrial knowledge and institutional experience. The sector can be considered mature, and the trends relatively stable. This can be good for future planning, and it can allow time for things like novel bioprocessing technologies to shake out glitches as they make it through the R&D and the regulatory pipeline. Of course, investors may not get nearly as excited about the molasses-speed of new product introduction, compared with other industries where inventions can make it to the market in months, rather than years. But the situation is much the same in the broader drugs (chemical substances as APIs) sector.
On the other hand, the bioprocessing market segment has grown remarkably consistently for the past 15 years we’ve been measuring supplier performance, at between 12-15 percent annually. Even when the data are broken out by growth in services, materials and equipment, the expansion shows notable stability; a good environment for risk-averse investors. This also matches the rate of growth in sales of biopharmaceuticals globally, as well. The upside of a highly regulated market that includes slow-motion trends is that its environment is relatively easy to track over time, and the data can be used to assess future movement more accurately.
This article presents trends and findings from BioPlan’s 15th (2018) Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, and discusses the impact of some of our findings. This survey and study provides a composite view and trends analysis from 222 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations in 22 countries, and includes over 130 direct suppliers of materials, services, and equipment. This year’s study covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time and assesses differences in the major markets in the U.S. and Europe.
Top Trends and Their Impact
Despite the overall, slow-motion nature of the biopharma industry, the significance and impact of events most definitely affect decision-makers, strategy development and investments in new facilities and technologies. Because decision-makers are affected differently by diverse shifts and trends, there are no real “top trends,” but rather, the trends have broad impact on the biopharma environment and how the industry responds.
The Importance of Manufacturing Efficiency and Productivity
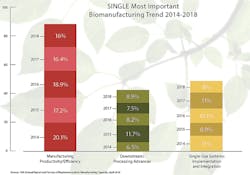
This year when respondents were asked to choose the “single most important trend or operational area,” the largest portion of respondents, 16 percent, cited “manufacturing productivity/efficiency,” with this largely unchanged from last year. Over the past six years, we have consistently seen a high interest this area. This affects virtually all stake-holders. Suppliers need to gear their new technologies toward making biologics cheaper, better and faster. Biopharmas need to plan for the future now, and must decide if the facilities and equipment they own or specify will be future-proofed. Will what they do be efficient and still operating well enough a decade from now, using then likely legacy, inefficient technologies?
Bioprocessing Productivity Continues to Increase
This year, the average titer reported at both clinical and commercial scales was 3.20 g/L. Annual survey data and other sources confirm that bioprocessing efficiency and productivity, in terms of upstream titers and downstream yields, will continue to increase. BioPlan has reported rather steady increases in bioprocessing productivity, particularly upstream bioprocessing, over the past 30 plus years, since the first adoption of recombinant technologies.
Biosimilars Bringing More Products and Players
Biosimilars/biogenerics are bringing many new bioprocessing players and facilities; and with these products facing considerable competition, cost-effective manufacturing is a basic requirement. The Biosimilars/Biobetters Pipeline Database reports more than 1,000 biosimilars (including biogenerics) in development or marketed worldwide. There are now over 300 biogenerics marketed in lesser- and non-regulated commerce in developing countries, each of which could perhaps be upgraded for major market entry. Over 750 companies are involved in follow-on (biosimilar, biobetter and biogenerics) products, with many new entrants in both developed and developing regions.
Facility Constraints Create Bottlenecks
The factor most frequently cited (50 percent) as likely to cause capacity constraints at respondent facilities in five years (2023) was “facility constraints.” This has remained the No. 1 cited factor since starting to ask this question in 2008.
“Develop better continuous bioprocessing — downstream technologies” was the area most commonly cited, by 42.2 percent as needing to be addressed to address (fix or avoid) future capacity constraints. Continuous downstream processing is also cited in responses to several other questions as needed and expected to resolve many of the current problems, with purification operations being the primary bottleneck in bioprocessing.
Lowering Manufacturing Costs
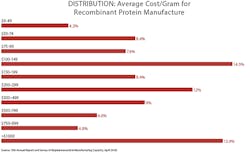
A majority of respondents continue to report efforts seeking to reduce bioprocessing costs, with 64 percent reporting that they have “implemented programs to reduce operating costs” at their facility within the past 12 months. Working to reduce bioprocessing costs has become a routine activity. This was the first year we surveyed about the cost/gram for recombinant protein manufacture. The average reported cost was $306.8/gram for respondents’ primary recombinant protein product, usually a monoclonal antibody.
Supplier Growth
Besides overall annual growth in the industry generally being above 12 percent over the past 20-plus years, the equipment and supplies sector is reporting health and even better growth. Surveyed supplier staff reported an average of 13.7 percent sales growth. “Equipment and instrumentation” took the top spot for growth with 16.8 percent average growth. Average growth reported in “raw materials and consumables” was 13.3 percent and “services (e.g., CMOs, CROs, consultants)” was 12.1 percent.
Bioprocessing Budgets are on the Rise
This year, no budget decreases were reported in any of the areas surveyed. Budgets for new capital equipment continued to be an area of significant growth, with respondents reporting an average increase of 8.2 percent in facility bioprocessing budgets for 2018. Much of this involves construction of new facilities, retrofitting, and the addition of capacity at existing facilities, (with an increasing number turning to single-use systems).
Single-Use Systems are Still the Rage
Again this year, over 90 percent of respondents reported currently using single-use equipment, with “tubing or disposable operations” alone cited the most, (90.6 percent), followed by “disposable filter cartridges,” (86.2 percent) and “bags, empty,” (81.8 percent). About 77 percent reported the use of single-use bioreactors. BioPlan estimates that more than 85 percent of pre-commercial product manufacturing now primarily involves single-use systems.
The Rise of Capacity in Asia
BioPlan’s free database ranks the top 1,000-plus biomanufacturing facilities worldwide. The current breakdown of worldwide bioprocessing capacity includes:
- US/Canada: 6 million L (37 percent)
- W. Europe: 5.5 million L (33 percent)
- Asia Pacific: 4.7 million L (25 percent)
- China: 876,000 L
- India: 833,000 L
Overall, the fastest growth (from low baselines) is in Asia, particularly China.
China Becoming an Industry Leader
In a survey of 50 biopharma executives in China by BioPlan staff, the largest portion, 58 percent, cited a “more innovative biopharma pipeline” as what China must possess to expand globally. This was followed by 50 percent citing both need to develop an “overall quality image” and “capacity, commercial scale.”
China has recently moved ahead of India in terms of bioprocessing capacity. Also, BioPlan’s directory of the top 60 biopharma manufacturing facilities in China portrays an embryonic industry working to expand its GMP-quality manufacturing capacity to supply domestic needs while also aiming to becoming a major global player in innovative and follow-on biopharmaceuticals.
Impacts of Trends
Collectively looking at its past, current status and trends, the biopharmaceutical industry is very healthy, and continues to grow at its usual rather steady pace, with most growth-related parameters, such as industry revenue, generally annually increasing by about 12 percent or more. In fact, there are hardly any significant negative trends. Despite any industry-related growth rates below the norm considered by some in the industry to be negative growth or contraction (with about 12 percent growth so long the norm, it’s considered the baseline), the roughly 12 percent annual biopharma industry growth rates are considerably higher than for most other established industries and the growth rates of developed countries. In the meantime, biopharmaceutical industry-related growth rates are even higher in the rest-of-the-world, particularly in the major Asian bioprocessing markets, although growth has only recently started from much lower or even near zero baselines.
The trends show that biopharma manufacturing is continuously growing, evolving, demanding and adopting (albeit slowly) new and improved technologies to reduce costs, increase efficiencies, improve product quality, and improve development pipelines. Ongoing industry trends support an optimistic vision of the future that includes more:
- Biopharma facilities worldwide, in both major markets and Asia
- Biopharma products
- Revenue and added-value for patients from innovations in products and processes
- Diversity in products in development and marketed, e.g., cellular and gene therapies
- Follow-on products and manufacturers, including biosimilars and biogenerics
- Flexible manufacturing facilities, including use for manufacture of multiple products
- Adoption of single-use systems at pre- and clinical scales, as well as for commercial manufacture
- Efficient bioprocessing — titers and yields will continue to increase
- Use of continuous processing, including for downstream processing
- Automation, monitoring and process control
- Use of bioprocess modeling, data mining, PAT, QbD
- Use of high-tech expression systems and other genetic engineering advances
- Modular facilities, including cloned facilities in developing countries and for regional cellular/gene therapies manufacturing
- Complex regulations which drive many other specific needs, advances and trends
What the Future Holds
The current situation in the biopharmaceutical industry is exciting, with new technologies and markets, such as continuous processing, biosimilars, cellular and gene therapies, and many new opportunities in emerging markets causing constant changes that must be adapted to.
Overall, the primary impetus for trends in bioprocessing is innovation. Innovation opens new opportunities and makes existing ones more attainable. It also speeds discovery, increases choices/options, and can drive down costs and improve productivity. The industry will see some (relatively) rapid changes in the coming years, including the rise of new classes of products, notably cellular and gene therapies; and expanded adoption of new(er) technologies, such as continuous bioprocessing. There will also be regulatory changes, such as more in-depth risk assessments related to leachates/extractables and other potential bioprocessing-associated risks to patients and staff.
However, changes in the bioprocessing sector, particularly any major advances and shifts in bioprocessing, take a long time. For example, it has taken over a decade for single-use systems to fully dominate pre-commercial biomanufacturing, despite the cost savings over stainless steel. Because new technologies are generally only adopted for new products, major changes in bioprocessing are inherently slow, generally take a decade or more. In the meantime, incremental innovation in improved manufacturing productivity continues. This ongoing adoption of new technologies, not dramatic paradigm shifts, is the general rule; and is the primary driver for many biopharmaceutical trends.
[javascriptSnippet]