Mitigating the Fall: Profitability Beyond the Blockbuster
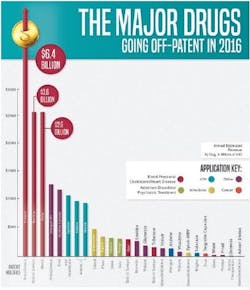
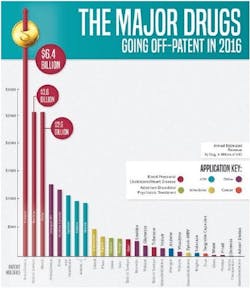
Even though the pharmaceutical industry is approaching what most analysts say is the tail end of the patent cliff tumble, the industry is still under pressure to develop strategies to stay profitable in what looks to be a new, non-blockbuster-reliant era.
Financially speaking, the good news for the pharmaceutical industry is that prescription (both branded and generic) drug sales continue to rise. EvaluatePharma predicts that the market for prescription drugs, based on consensus forecasts for the leading 500 pharmaceutical and biotechnology companies, will grow by 4.8 percent per year to reach $987 billion by 2020.
Branded pharmaceutical companies have been employing a combination of strategies, often concurrently, to retain market share once generics come into play. Forward-thinking companies have adjusted their business strategies, finding ways to turn dreaded patent expirations into opportunities to innovate and bring new value to patients and populations.
MARKET PROTECTION FUNDAMENTALS
At the most basic level, drugs have two forms of market protection in the U.S. — exclusivity and patent protection. Patents, granted by the U.S. Patent and Trademark Office, generally have a term of 20 years from the date of filing. Beyond the initial patent filings, which protect compositional matter of a new chemical entity, secondary or “follow on” patents can be sought to protect improvements to, additional discoveries through scientific data, or new uses for the pharmaceutical not suggested in the original patent.
Peter Knauer is chief regulatory officer for ARC Experts, a leading consultancy offering specialized services to the pharma, medical device, diagnostics and biotech industries to assist with regulatory, compliance, audits and all risk related issues. Knauer, a veteran of nine successful NDA/BLA approvals, explains, “There is essentially a ‘porfolio’ of patent opportunities that come with each product, so manufacturers can extend patent life years beyond initial patent. But eventually, even that runs out, so where do you take it from there?”
The answer to that for many manufacturers often lies in exclusivity. Given the complexity and length of the drug approval process, there is often little patent protection left on a product by the time the drug hits the market. To provide pharmaceutical companies with a fair chance to recoup R&D investments and incentivize continuing innovation, the Food and Drug Administration offers numerous exclusivity provisions to drug manufacturers. The FDA cannot legally approve a generic drug application for that product until the exclusivity period expires.
“There are unique opportunities that can offer additional and extended exclusivity to encourage a pharmaceutical manufacturer to go after smaller markets, and getting this exclusivity can be very lucrative for a company,” says Knauer.
One trend that continues to gain favor, according to Knauer, is seeking orphan drug exclusivity. Targeted at diseases with high unmet medical needs, orphan drugs have the potential to receive faster approval from the regulatory agencies and higher levels of reimbursement. Companies can request orphan-drug designation of a previously unapproved drug, or of a new use for an already marketed drug — breathing new life into an already existing product.
If a product is granted orphan drug exclusivity, FDA may not approve applications for generic products that contain the same active ingredient and are labeled for the same orphan indication for seven years. The market for orphan drugs, according to EvaluatePharma’s 2015 Orphan Drug Report, will grow by 11.7 percent per year between 2015 and 2020 to reach $178 billion. And, according to the report, “large pharma groups finding orphan indications for some of their biggest sellers mean that seven of the 10 top companies by orphan indications are global majors.”
STRONGER FOCUS ON LIFECYCLE MANAGEMENT
The post-blockbuster era has led to a fundamental shift in how pharmaceutical manufacturers structure their organizations, with more attention being given to the optimization of existing branded drugs. The majority of branded drugmakers now have dedicated business units for managing established products.
Extension strategies such as reformulating drug delivery or finding new indications are common in pharma lifecycle management. Critics of pharma lifecycle management often cite reformulation in the “evergreening” debate, claiming that drug companies are more focused on their own economic value than the therapeutic value these extensions bring to patients. Recent political focus on drug pricing has seen policy makers pushing the FDA to target exclusivity periods to only the truly innovative products, rather than drugs that are minimally different from existing ones.
As a result, many branded pharma manufactures are re-evaluating their product lifecycle management strategies in order to best maximize products still under patent protection and provide true patient value. According to Goeller, “Which lifecycle management approach companies take always depends on how they feel they can best build a value position in the market. To be successful, manufacturers need to offer product extensions that bring true clinical benefits that improve the quality of life for patients.”
Catalent, for example, is helping its customers develop new drug-delivery formats that overcome a major formulation obstacle — poor solubility. Solubility is one of the most frequent culprits of poor bioavailability and limited drug absorption. According to Chris Halling, senior manager of global communications for Catalent Pharma Solutions, “Solubility of existing drugs is an issue that is often overlooked in favor of developing new drugs.” Catalent has extensive experience converting existing formulations into other forms such as softgels, which may offer a solution to solubility challenges, as well as patent benefits and numerous patient experience benefits. Softgels are perceived to generate a faster onset of action, can reduce API dose and side effects, and improve overall treatment performance. Additionally, softgels encourage compliance, as they are sometimes easier to swallow, appealing to specific patient groups, such as the elderly.
These newly realized benefits become extremely important when looking to product lifecycle management as a patent extension strategy. Echoing the sentiments of Goeller, Halling says, “It is not worth doing unless you can truly differentiate your product and show patients a genuine brand value.”
BETTER LATE THAN NEVER?
It is universally acknowledged that a crucial component of product lifecycle management in general is timing — establishing a plan for patent expiration mitigation early in a product’s lifecycle. The unfortunate reality is that this rarely happens.
The extensive and exhausting process involved in getting an initial drug approval as well as managing existing products oftentimes means anticipating the patent expiration from the drug development stage is little more than “wishful thinking.”
“Today’s pharma companies are operating lean and are busy nurturing existing products and managing risk strategies,” says Knauer. “As such, being able to not only plan, but then strategically implement forward thinking product lifecycle extending or enhancing plans is challenging.” Knauer has offered several ideas around dovetailing improvement and enhancement of intellectual property and patent management plans:
1. More transparent licensing or grant options on the existing library of compounds, with exclusivity, to boost innovation and speed the process of drug discovery and development.
2. More comprehensive and deeper understanding of all international regulatory compliance additions and changes.
3. Development of collaborative academic-to-industry relationships to boost innovation and Intellectual Property generation.
4. Assess potential of emerging markets early, i.e., China, India, Middle East, Africa to understanding their challenges, demographics, specific diseases and resources available.
5. Encouraging patent reform, by working with foreign patent authorities in developing countries, for protection of their products through patent exclusivity enforcement.
Some pharmaceutical companies are finding ways to get ahead by researching extension options well in advance of patent expirations.
Novo Nordisk, for example, is looking to minimize loss after its blockbuster injectable type-2 diabetes medication, Victoza, loses patent protection next year. Novo is approaching commercialization of replacement biologic, semaglutide and recently announced results from the last global Phase 3a trial. Semaglutide is a once weekly injectable for the treatment of type II diabetes. Novo recognizes, like many branded pharmaceutical manufacturers, that reformulation of injectables into orals can extend the lifecycle value of existing molecules. Already ahead of the game, Novo is simultaneously developing a long-acting oral version of semaglutide intended as a once-daily tablet treatment for the same indications as the injectable version. Currently, GLP-1 receptor agonists are available only as injectables.
OUTCOMES-BASED APPROACH
The definition of “value,” however, is subjective and varied, making this a difficult model to adopt. What can be derived from this, though, is the idea that drug pricing should have a close relationship with the drug’s ability to deliver results. Though the idea is still in nascent, some branded drug manufacturers are responding to this call-for-action by experimenting with pay-for-performance models tied to a treatment’s effectiveness rather than simply the volume of drugs sold. These drugmakers are putting resources in place from the start of the process to ensure molecules are developed in a way that truly delivers value, in order to counteract the limited days of the blockbuster.
In 2013, Novartis CEO Joseph Jimenez spoke candidly about how he navigated Novartis through the loss of patent protection on its best-seller, Diovan. Said Jimenez in a Wall Street Journal interview, “I really believe that in the future, companies like Novartis are going to be paid on patient outcomes as opposed to selling the pill.” Though Jimenez has recently addressed the obstacles Novartis faced when trying to implement an outcomes-based plan for its heart drug Entresto, Jimenez is still supportive of a future industry shift away from a transactional approach to pricing and selling drugs.
Amgen saw better success with its outcomes-based approach taken with pricey cholesterol drug, Repatha, approved by the FDA in mid-2015. Amgen linked the net price of Repatha to expected LDL cholesterol reductions and anticipated appropriate patient utilization. Unique to the deal that Amgen set up with insurers is that Amgen will have to provide larger rebates to payers if patients’ cholesterol levels are not lowered to levels observed during clinical trials.
An outcomes-based approach has the potential to make insurance companies and government payers more willing to approve reimbursement for new drugs, as well as help drugmakers to differentiate their pharmaceuticals against competitors through higher value achievement.
EMERGING MARKETS
According to McKinsey analysis, between 2015 and 2020, emerging pharmaceutical markets are expected to account for $190 billion in sales growth. Generics play a pivotal role in these markets. “Sometimes the solution comes down to geography. Generics behave differently and have different regulations in emerging markets,” notes Goeller.
While entering the unbranded commodity generics market in the U.S. — where generics are sold at the lowest possible price and most consumers cannot distinguish one manufacturer from another — is not vastly appealing to branded pharma; generics in emerging markets such as Russia or China offer more potential.
“The U.S. has rapid generic erosion, whereas in many emerging markets the originators are better able to retain market share,” says Goeller.
In these countries generics are typically sold as branded products — sometimes for as much as 80 percent of the original price. These markets are often plagued with quality issues, resulting in consumers who are more likely to opt for generics that carry the name of a trusted manufacturer.
Despite existing obstacles surrounding healthcare infrastructure and IP protection, branded pharmaceutical companies are recognizing the value in these emerging markets, and customizing their strategies in order to create a product that is accessible to the masses and yet profitable at the same time.
As the pharmaceutical landscape changes, branded drugmakers are shifting their focus from big money blockbusters to strategies that increase returns from already approved drugs, before and after patents expire. What’s old can in fact be new again, and in some cases, yield more effective treatments and meet the unmet medicals needs of a wider population.
About the Author
Karen P. Langhauser
Chief Content Director, Pharma Manufacturing
Karen currently serves as Pharma Manufacturing's chief content director.
Now having dedicated her entire career to b2b journalism, Karen got her start writing for Food Manufacturing magazine. She made the decision to trade food for drugs in 2013, when she joined Putman Media as the digital content manager for Pharma Manufacturing, later taking the helm on the brand in 2016.
As an award-winning journalist with 20+ years experience writing in the manufacturing space, Karen passionately believes that b2b content does not have to suck. As the content director, her ongoing mission has been to keep Pharma Manufacturing's editorial look, tone and content fresh and accessible.
Karen graduated with honors from Bucknell University, where she majored in English and played Division 1 softball for the Bison. Happily living in NJ's famed Asbury Park, Karen is a retired Garden State Rollergirl, known to the roller derby community as the 'Predator-in-Chief.'