Respondents to Nice Insight’s annual survey of outsourcing-facing pharmaceutical and biotechnology executives expect spending on outsourcing to increase for the fourth year in a row. This result fits well with estimates that the global contract pharmaceutical manufacturing market is growing at an average annual rate of 7.5%. There are many factors driving this growth in 2016 — increasing consumption of medicines around the world; a more robust pipeline of drug candidates and an increasing rate of FDA NDA/BLA approvals; the increasing focus on biologic drugs, including by traditional pharma companies that lack biotech expertise; the entrance into the market of numerous small, virtual startups that have no manufacturing capacity; the rise in patent expiries and increasing generics competition, which is driving a greater need for cost efficiencies and access to novel, proprietary technologies for achieving product differentiation; and the increasing complexity of both small- and large-molecule drugs such as antibody-drug conjugates and highly potent compounds.
DRAMATIC INCREASE IN SPENDING
This strong growth is supported by the results of Nice Insight’s 2016 CDMO Outsourcing annual survey of professionals in the pharmaceutical and biopharmaceutical industries; participants have indicated that their companies have dramatically increased year-over-year spending on outsourcing for the last four years.
Survey results also indicate that further spending can be expected. Nearly 95% of respondents expect that their companies will either maintain (18%) or increase (75%) their spending on contract development and manufacturing services over the next five years. Furthermore, while three-quarters of respondents currently use 0-10 CDMOs and/or CMOs, 7% use 11-20 and 5% use 21-30, 69% of participants in the 2016 CDMO survey expert to increase the use of CDMOs and CMOs going forward, with 29% expecting the number of manufacturing partners to remain the same, and only 1% expecting to decrease the number of partners.
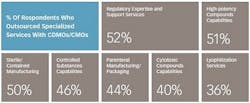
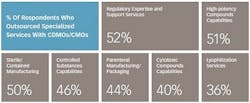
These numbers reflect the robustness of pharmaceutical pipelines and an overall greater need for support. Slightly more than half (56%) of respondents to the 2016 Nice Insight CDMO Outsourcing survey indicated that an expanding R&D portfolio is driving their increasing use of CDMOs and CMOs. Survey participants also indicated that their companies are increasing the use of outsourcing as part of their manufacturing strategies (60%) due to the need to address patent life issues, the need for novel delivery forms and other specialized capabilities, and a desire to increase decentralization for greater flexibility. Companies are also expanding the use of service providers because they have had positive experiences with outsourcing to CDMOs and CMOs in the past (59%).
Interestingly, outsourcing is not highly focused at any one particular development stage, although the percentage of respondents outsourcing Phase II projects (63%, up from 42% in 2014) to CDMOs and CMOs is slightly higher than those using manufacturing services for Phase III (54%, up from 29% in 2014), Phase I (53%) and pre-clinical, including discovery phase, (51%) projects. The distribution is fairly even, however, and is another reflection of the robust drug pipeline resulting from significant investment in innovation; many candidate drugs are now steadily moving toward commercialization. Phase IV/Post-Launch projects are outsourced by 39% of survey respondents; the lower level of outsourcing compared to those at earlier phases reflects the attrition that occurs as safety and efficacy are evaluated. However, the number is nearly double that for outsourcing on Phase IV/Post-Launch projects in 2014 (22%). The much higher percentages of respondents outsourcing phase II, III and IV projects strongly suggest that new programs designed to weed out unlikely candidates as early as possible in the development process and well before they enter into clinical trials are achieving the desired results.
WHO WILL BE THE WINNERS?
Not all contract manufacturers will benefit from the increased spending anticipated by the participants in this year’s Nice Insight CDMO Outsourcing survey. Indeed, the percentage of respondents that elect to work with “Preferred Suppliers” rose to 43% from 35% last year, while the preference for tactical suppliers dropped from 35% to 31%.
What does it take to be preferred? Such firms tend to have more global footprints, large-scale capabilities for greater cost efficiencies, a broad range of service offerings including development and final formulation/drug delivery in addition to API manufacturing, and access to advanced technologies. In particular, successful CDMOs make it possible for drug manufacturers to more efficiently and cost effectively develop and produce increasingly complex drug candidates and extend the product lifetimes of off-patent products using novel, proprietary technologies. Consequently, technological capabilities will equate directly to competive advantage as drug manufacturers continue to pare down their vendor numbers and establish preferred/strategic partnerships with fewer, integrated suppliers.
It should also be noted, however, that preferred suppliers must continually earn their position with ongoing high levels of performance. For instance, half of the 2016 Nice Insight CDMO Outsourcing survey respondents indicated that they would switch CDMOs if they do not continually meet quality and on-time delivery expectations.
So where will the opportunities lie for CDMOs in 2016? Companies with truly integrated offerings and unique technical capabilities will enter into collaborative capacity management and long-term, multiproject relationships. Emerging markets, value-added generics (so-called supergenerics), and biosimilars will provide other potential opportunities for growth. Those that are positioned to leverage these opportunities will survive the more competitive contract manufacturing marketplace.
To learn more about Nice Insight, contact Guy Tiene at [email protected] or visit www.niceinsight.com.