Freeze drying is a process comprised of three main steps: freezing (solidification), primary drying (ice sublimation), and secondary drying (moisture desorption), and can take several days to accomplish. The overall efficiency and consistency of the entire process, as well as ensuring high quality of the products, largely depends on the nucleation temperature. Temperature directly affects the size of ice crystals which, in turn, determines the pore size distribution, and therefore, resistance of the porous freeze-dried matrix to vapor flow.
FIRST, MEET THESE TWO CONDITIONS
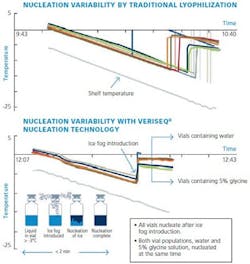
Figure 1: Nucleation Variability
In order for nucleation to occur, two process conditions must be met: the product temperature has to be lower than the freezing point of the solution and there have to be nucleation sites present to trigger the process. The temperature difference between the equilibrium freezing point and the ice nucleation point is known as super-cooling. A lower nucleation temperature, or a higher degree of super-cooling, results in more ice-nuclei and smaller ice crystals. On the other hand, higher nucleation temperature, or a lower degree of super-cooling, results in fewer ice-nuclei and larger ice crystals, which eventually form pores and pore networks. Larger pores enable higher sublimation rates, hence shorter drying cycles, as well as reduced reconstitution times and improved finished product attributes. It is also important that all vials nucleate at the same temperature, to ensure consistency of the product morphology, resultant cake structure and appearance, as well as uniformity of the product from vial to vial. However, in absence of nucleation sites (uncontrolled conditions), which is very common in the case of smooth-wall sterilized glass vials, the spread in nucleation temperatures between different vials, and hence, non-uniformity of the final product, could be quite significant.The freezing step, therefore, is one of the most important steps in the lyophilization process. Handling freezing in a “controlled” versus “uncontrolled” or “random” fashion results in a number of benefits to the product manufacturers as well as end-users.Products that could benefit from implementation of controlled nucleation include: biological products like protein and peptide formulations, vaccines, liposome and small-chemical drugs susceptible to physical and chemical degradation, as well as other injectables, which must remain effective from manufacture to patient administration [2]. Due to the rapid growth in biological therapies, the demand for freeze drying has never been higher, and this trend is expected to continue for the next decade [3].
Developed by Linde Gases in cooperation with IMA Life North America, the VERISEQ Nucleation technology offers a commercially viable technique for cryogenically generating a uniform dispersion of microscopic ice crystals (or ice-fog). Upon introduction into pre-cooled vials (or seeding the vials), containing the product to be freeze dried, these ice-fog crystals serve as nucleation sites. This causes a rapid and uniform nucleation of the product in a vial as well as between vials of the same batch at very low degrees of super-cooling [5].
Hence, the ice-fog introduction follows a two-step approach: the product-containing vials are first cooled to a selected suitable temperature at or below their freezing point, and then, the ice-fog is introduced to facilitate nucleation. A key challenge for Linde technology was to generate a sufficient amount of ice-fog to fill the chamber and to ensure its penetration inside the vials given various lyophiliser volumes and vial/stopper geometries. This was achieved by implementing a proprietary mixing assembly, which provided an extremely efficient mechanism of quickly forming the ice-fog and circulating it throughout the freeze chamber. Linde’s nucleation system has no moving parts or other complicated components that would be difficult to steam or otherwise sterilize. It requires no chamber pressurization and can be retrofitted to practically any freeze dryer.
Though there are many approaches to achieve controlled nucleation, only few techniques can do so on a commercial scale. Ice-fog technology can be effectively utilized in laboratory and/or production-scale lyophilisers to induce uniform ice nucleation at reduced levels of super-cooling and eliminate vial-to-vial variability, which in turn can help mitigate a host of related issues and lead to improved process parameters as well as product quality.
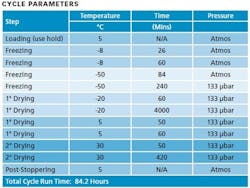
Table 1
Monoclonal Antibodies (mAbs) comprise an exciting array of new therapies for oncology, infectious disease and organ transplant to name but a few. Their promise is reflected by continued increase in both capital investment and regulatory approvals. As with many other proteins, maintaining stability in the liquid state is difficult. For this reason, lyophilization is used to stabilize most mAbs formulations for prolonged shelf life. The lyophilization process is lengthy, expensive, and can be risky as equipment failures can lead to the loss of entire batches.Exacerbating the length of the process is the relatively high concentration of material required for mAb therapies (in most cases between 90 and 125 mg/ml). Although lyophilization is a batch process, variability between units is common, due mainly to differences in freezing behavior, with other factors having a lesser impact later in the process. Efforts to optimize the lyophilization cycle can increase the non-homogeneity of product batches due to non-uniform drying of units in the batch.Product freezing subjects units to liquid super cooling: the temperature depression of a liquid below the freezing temperature before ice forms, which is triggered by nucleation. Nucleation temperature will advise crystal formation to form the vapor pathways for subsequent drying. Because each unit in the batch nucleates individually and in many cases at different temperatures from one another (uncontrolled nucleation), there can be a great deal of difference in the drying rates between individuals.The study was designed to evaluate the impact of controlled nucleation on the ability to safely optimize a lyophilization cycle for a model mAbs formulation, as well as to evaluate the impact on finished product attributes.Materials and equipment:
• Product type: Monoclonal Antibody
• Approximate solid content: 100mg/mL
• Collapse Temperature: Tc= -17.3°C (Initial Collapse), -15.7°C (Total Collapse)
• Glass Transition Temperature: Tg’=-25.83°C
• Fill volume: 1 mL in 2mL borosilicate vial with 13mm igloo stopper
• Filling performed in Class B Clean Room
• Lyophilizer: IMA LyoFast 3 (2.3 m2)
• Controlled nucleation device: Linde VERISEQ® Nucleation unit
• Capacity: 200 Product vials processed for each batch
• Temperature sensors placement: Thermocouples placed inside and on outside of vials, and scattered throughout vial pack
TEST PROCEDURE
Two hundred vials were loaded into the lyophilizer and processed using the cycle provided. The process was monitored using product thermocouples, as well as differential pressure measurement obtained using a capacitance manometer and a Pirani gauge. Finished vials were sampled for residual moisture, reconstitution, and physical micro-structure.
Nucleation ice fog being applied to product vials.
This process was repeated for a second batch of 200 vials, with the only difference being the introduction of ice fog to trigger nucleation in each vial. This occurred at the end of the -8°C hold step. The ice fog was generated using the Linde Nucleation unit, which uses clean steam and sterile liquid nitrogen to generate a cryogenic fog which is injected into the lyophilizer chamber at sub-atmospheric pressures, resulting in a dense, well-distributed fog (see Figure 1). When the fog is injected with the product stabilized at a given temperature, nucleation occurs in each vial at the same temperature, meaning that the batch should dry in a faster and more homogeneous manner.This experiment demonstrates the impact of controlled nucleation on drying time. The second trial, with all elements of the product and cycle identical to the first, showed a 16:40 hour reduction in primary drying through via the Linde nucleation technology. In addition, faster sublimation causes lower product temperatures in the second cycle. This implies that more aggressive cycle conditions would be feasible when controlling nucleation for this product. Finally, the steeper slope of the Pirani curve indicates that vials nucleating at the same temperature tend to dry at similar rates, with far less variability than indicated by the baseline curve.
Based on the cooler thermocouple temperatures noted in the controlled nucleation trial, an attempt was made to optimize the process by both raising the shelf temperature from -20°C to -8°C and shortening the primary drying time. Drying time was completed at 1,360 minutes, an additional reduction in drying time of 14:50 hours.
PRODUCT ANALYSIS
Table 2
Each trial batch was sampled for residual moisture (5 samples per batch) as determined by Karl Fisher Titration, and reconstitution time. Table 2 summarizes the results.Clearly the study indicates that controlling nucleation can lead to shorter drying times, more consistent behavior within a population of vials and enhances finished product characteristics. Because of the enhanced homogeneity of the vials, and the faster rate of sublimation, more aggressive processing conditions can be used. In this case, nucleation control is easily achieved through the integration of Linde’s nucleation system with a commercial lyophilizer.
REFERENCES
1. Siew A. (2013) Lyophilisation technologies for controlled nucleation. Pharmaceutical Technology 37(5):36-40
2. Gupta A. (2012) Short review on controlled nucleation. Int. J. Drug Development & Research, 4(3).
3. T2+2™ Market Overview Freeze-Drying for Biotechnology and Pharmaceuticals (24 July, 2009). http://www.foresightst.com
4. Thomas P. (2011) Controlled Ice Nucleation Moves into Manufacturing: Interview with Mike Pikal (University of Connecticut). http://www.pharmamanufacturing.com .
5. Chakravarty P., Lee R., DeMarco F., and Renzi E. (2012) Ice-fog as a means to induce uniform ice nucleation during lyophilisation. BioPharm International 25(1):33-38.