It’s probably fair to say that innovation in the biopharmaceutical manufacturing industry is a slow cycle. But that would ignore the many changes in biomanufacturing over the past 5 to 10 years: Better expression systems, widespread adoption of novel single-use applications, re-emergence of perfusion technologies, new modular and flexible facilities, better sensors, control systems and downstream technologies.
The industry now finds itself very aware of the promise new technologies carry in terms of optimizing, and in some cases revolutionizing existing processes. Results from our “10th Annual Report and Survey of Biopharmaceutical Manufacturers”1, in which we surveyed 238 biomanufacturers, indicate that end-users are still actively looking for a range of new technologies to solve persistent problems.
PRODUCTIVITY INNOVATION 2013
In a separate survey Bioplan Associate’s ran late last year, among the more than 450 global subject matter experts and senior participants who make up our Biotechnology Industry Council,2 the study found consistent expectations regarding improvements in productivity. Again, improvements in downstream processing and single-use technologies ranked as the top 3 trends for 2013, these were followed by demands for better analytical methods. New analytical methods are required for better process monitoring and process improvements. In addition, to develop biosimilars, the industry needs better characterization techniques, and better processes. Otherwise, even if “similarity” with a reference biologics could be shown, the cost of producing a new biosimilar might not be much lower than the original; this could dramatically reduce the attractiveness of any such high-cost generic version.
Returning to the attractiveness of single-use technologies, we found in our annual survey that respondents today estimate 35% of their upstream clinical production operations to be single-use. This compares with 25% of respondents that said more than 80% of their downstream clinical production steps are now single-use. The number is 16% of downstream commercial-scale production. This wasn’t surprising to see that the lowest use was for downstream commercial production, which remains mostly fixed stainless-steel equipment. It was also fairly consistent to see the highest adoption rate be for downstream clinical production, likely due to broader use of disposable tubing and filters, buffer containers, etc.
FOCUS SUPPLIERS, FOCUS
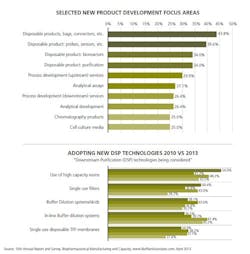
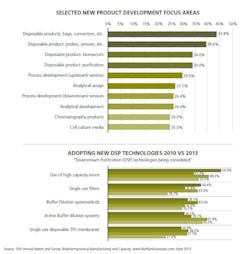
Researchers asked respondents to consider new product and services developed by suppliers and to identify the top areas they want suppliers to focus their development efforts on. This year, of the 21 areas the study listed, the areas highlighted in prior years, and other studies continue to occupy the top position. Specifically:
- Disposable products, including bags, connectors and other devices
- Better probes and sensors
- Process development services (up- and down-stream)
- Chromatography products
THE INNOVATION PENDULUM
Although bioprocessing industry growth isn’t as radical as in semiconductors (where Moore’s Law proposed that the number of transistors on a chip doubles every 18 months), demand for innovation that improves efficiency is similar in respect to how new product developments swing from one bottleneck to the next. This year, single-use applications are clearly the subject of much interest when it comes to innovation; in prior years, and likely in the future, other technology areas will bounce back. Downstream purification, new and better analytical tools, and improved services offerings for example are likely to re-emerge as urgent problems as the industry resolves current, more acute, issues.
For example, separately in our study we asked respondents which of 21 different, novel downstream purification (DSP) technologies they were actively considering to address bioprocessing problems (see Figure 2). The responses are indicative of potential future adoption and do not take into account respondents already having adopted these technologies or those who are considering but not actively pursuing them.
WHAT’S AHEAD FOR NEW TECHNOLOGIES
The biopharmaceutical manufacturing industry is actively considering and adopting a range of new technologies for all facets of the manufacturing process. Single-use devices continue to crop up in any conversation about new technologies and a move towards leaner, more flexible and “modular” systems seems likely in biopharma’s future.
It is also important to note that introduction of innovations is not at all easy in this industry, and there are many obstacles to new technology introductions. Regulatory issues force biomanufacturers to stabilize bioprocessing systems early on, so processes can remain largely unaltered through a drug product’s lifetime. This can make manufacturers less receptive to improvements. Thus, manufacturing strategy takes a long view when it comes to adoption of innovation.
On the other side, vendors, larger ones in particular, invest significantly in R&D and product lines. They have a vested interest in evaluating where future adoptions will be needed, and how rapidly they will be taken up.
Economic conditions can also play a role. While budgets are again expanding, tighter conditions continue to discourage the financing — and entrance — of smaller suppliers in the market, reducing the pool of likely contributors to innovation. Even so, biomanufacturers are showing a renewed urgency to improve productivity, reduce costs while boosting quality. This is reflected in increasing budgets over the past four years supporting activities focused on production efficiency.
Given the complexity, and the long product development cycle, the only way to ensure efficient process is for industry suppliers and vendors to continue to identify and meet the demands of end-users. This will drive future investing in the development of new technologies. And supporting this, on the suppliers’ side, we find that vendors’ R&D budgets for new product development have also expanded over the four years.
So, with increased budgets and interest from both manufacturers and vendors on innovation that boost efficiency, it’s easy to visualize a robust future for the industry. For biomanufacturers to truly push forward innovation and remain competitive as cost pressures increase, and biosimilars evolve, they will continue to demand better ways of evaluating new technologies to cut downtime to market and streamline the overall testing process.
Survey Methodology: The 2013 “10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production” yields a composite view and trend analysis from 238 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 30 countries. The methodology also included over 158 direct suppliers of materials, services and equipment to this industry. This year’s study covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world’s major markets in the U.S. and Europe.
Published in the November 2013 edition of Pharmaceutical Manufacturing magazine
About the Author:
Eric S. Langer is president and managing partner at BioPlan Associates Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD, in 1989. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing”, and many other industry reports. Contact Eric at: [email protected]; 301-921-5979; www.bioplanassociates.com.
References
1 “10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production: A Survey of Biotherapeutic Developers and Contract Manufacturing Organizations,” BioPlan Associates, April 2013.
2 BioPlan Associates’ “2013 Biotechnology Industry CouncilTM Trends Analysis Study”