Managing Contract Partners: Do We Have a Failure to Communicate?
As competitive pressures increase, nothing can stop the pharmaceutical outsourcing juggernaut. What started off as a “back office” practice for business, IT, HR and real estate management has become well established in manufacturing, and the use of outsourcing continues to grow in other strategic functions, including R&D and clinical.
Developing an accurate assessment of the pharmaceutical outsourcing market’s size is nearly impossible, says Nigel Walker, managing director of That’s Nice (New York, N.Y.) whose Nice Insight market research program studies the evolving contract pharmaceutical services market closely.
A wide range of companies offer services from tiny, privately held and extremely niched players to generic drug manufacturers and even big pharma companies.
The market research company Frost & Sullivan estimates that the pharmaceutical contract services market is roughly $10.7 billion in the United States alone, and growing by roughly 8% per year [1].
While pharmaceutical manufacturing and development outsourcing has increased, so have pharmaceutical recalls and other regulatory issues, including 483s and consent decrees (Figure 1).
Observers say this parallel growth is no coincidence. Over the past five years, McKinsey & Co. consultants have found there has been a 16%/yr increase in pharmaceutical recalls that can be directly traced to quality failures on the part of suppliers or contract partners.
Paradoxically, in surveys, operating pharmaceutical companies say that quality is the top reason they select a contract partner. CMO’s ability to comply with regulatory requirements is another one of their top selection criteria. [2,3]
Regulators continue to emphasize the need for better risk management. “There has been an evidentiary shift that places the burden on the industry to prove appropriate levels of risk management,” said Michael Long, director of consulting services for Concordia ValSource, LLC (Downington, Pa).
In the future, he says, pharma supply chains may more closely resemble those of the automotive industry, with Tier 1 and 2 suppliers. However, in the short term, Long says, expect more questions from regulators surrounding risk management, product and process knowledge, he says [4].
Not only regulators, but observers and experts within the industry are calling for much stronger outsourcing governance [5]. The subject is complex, touching on risk management, staffing, training, tech transfer and communications. Are drug manufacturers ready for the challenge?
In this article, two industry experts comment on the issues playing out right now, and suggest best practices. The article also examines results of a recent reader survey, which sheds some light on how drug manufacturers are responding to the challenges of contract supplier oversight.
Risk Management 101
Clearly, many are at an early stage in developing risk management strategies. “Even though ICH Q9 was published six years ago, drug manufacturers are just starting to find their footing in the areas of risk management, quality by design, and quality systems,” said Long. Their progress, he says, depends on how advanced they are in applying risk management tools and concepts.
“If you do not have an adequate quality system in place, with adequate controls, all the product and process development and the process understanding in the world, may go to waste,” he told attendees at PDA’s annual meeting earlier this year in Phoenix. In addition, he says, some professionals have fundamentally misunderstood the concept of a “risk-based approach,” Long said. “It is not a gift card for reducing testing and other precautions,” he said. “Instead, it requires a balance between identifying and mitigating threats, while taking advantage of opportunities. It should never become a hammer in search of a nail, and all systems must be evaluated if it is to be robust.”
Managing contract manufacturers requires asking two key questions, according to Hedley Rees, consultant and founder of the U.K.-based consultancy, Biotech PharmaFlow, who established and chairs the Drug Industry Modernization group on LinkedIn and whose extensive book on optimizing pharmaceutical supply chain management was published two years ago [6]:
1) Do I understand the extent of my obligations to manage my CMOs?
2) Have I the right processes in place to deliver on those obligations?
All manufacture and testing carried out at third parties must be treated as if it were carried out by the drug manufacturer itself, Rees said, and the working supply chain must comply to regulations at every stage. This means:
- Investigating out-of-specification results and appropriate (root cause) corrective and preventive actions
- Examining complaints handling processes
- Reviewing technical documentation and ensuring that it is approved by suitably knowledgeable and qualified personnel
- Ensuring that supply and quality agreements are worded to provide maximum alignment between standard operating procedures (SOPs) across organizational boundaries.
“Contracts must closely spell out such widely ranging activities as corrective and preventive action (CAPA), technology transfer, operation of interfacing quality systems, specific mitigations emerging for risk assessments as well as newer approaches, such as the adoption of a pharmaceutical Quality by Design (QbD )approach,” Rees said. “If your contract did not spell out alignment, it may not happen.”
The best approach, he said, is to view outsourcing as a specific case of procurement, and to remember that it is a strategic, organizational function. Rees urges the following:
1) Identify and involve all stakeholders in the outsourcing process, from the start, and do not leave key issues to either the procurement department or the CMC group.
2) Beware of checklist Quality Agreements based on legal boilerplate. The commercial and technical terms for the agreements (Supply and Quality) must form part of the tender and the pre-contractual negotiations. Terms should be based on the practical ways you will work together to meet your mutual obligations.
3) Remember that power shifts after contracts are signed, especially if you are entering a single-provider arrangement. If anything important is left out of the contract, such as the requirement that certain information be provided by the contract company on a regular basis, then you will have no reason to expect it because the contract partner is under no obligation to provide it.
How Are We Doing?
Is the typical pharmaceutical manufacturer developing the right approach to CMO management? Pharmaceutical Manufacturing surveyed readers to get a snapshot of contract partner management practices. Over 173 industry professionals responded to the survey, results of which are highlighted below.
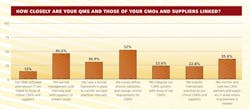
When asked how closely they synchronized their internal quality systems with those of CMOs and suppliers (Figure 2) 64% of respondents said they defined process validation and change control requirements closely for their CMOs; 46% said they used risk management tools internally and with suppliers.
In addition, 36% said they monitored and trained CMO partners in areas where improvement was needed, and 31% described having a knowledge transfer process available, to transfer internal best practices to their contract partners. Twenty-four percent said they had integrated CAPA systems with those of their suppliers.
Fewer respondents are using technology to facilitate connection to CMOs; 13% said they had connected QMS and other IT platforms to those of conract partners and suppliers.
As far as specific risk management tool kits and methods are concerned (Figure 3), 49% of respondents said they were using failure modes and effect analysis (FMEA), 43% are using process capability analysis, 40% are using Six Sigma, 38% say they use QbD, and 36% report using process analytical technology (PAT).
Eighty-six percent of respondents said they have a formal process in place to monitor the source and quality of raw materials critical to product quality bought by suppliers. (Figure 4) The remainder did not.
On the positive side, most respondents to the survey said they have a system in place for monitoring the quality performance of critical CMOs and suppliers. Sixty-one percent said they hold regular meetings with contract partners, 58% send senior quality staffers to visit supplier sites, 58% say they review relevant manufacturing and process monitoring data regularly; and 20% have set up dashboards to monitor KPIs for contract partners.
When asked to define their biggest challenges in managing CMOs, most respondents (30%) cited knowledge transfer; 24%, process validation; and an equal number, change control. In addition, 23% said that risk management was their top challenge, 21% reported monitoring, 15% CAPA coordination and 14% tech transfer.
“Someone always seems to be asleep at the switch,” wrote one. Another described high attrition rates at smaller CMOs, with poor knowledge transfer the result.
“If you don’t have a quality and technical rep on site for each batch produced at a CMO, there are items that don’t get documented at the same time, so resulting deviations aren’t always documented efficiently.”
Among other issues respondents cited:
- A lot of manufacturing and quality data for CMOs can only be seen during on-site visits
- CMOs need to prevent process drift and poor decisions by management
- Insufficient knowledge of CMC issues
- Wrote one respondent, “It can be difficult to ask informational questions from most of our suppliers. They are reluctant to provide helpful information for fear of incriminating their own products.”
Other respondents noted that, given limited internal resources, it was becoming more difficult to maintain close and meaningful contact with suppliers. Said another “review of documentation alone does not provide a full picture of actual performance.”
Communication and Knowledge Transfer
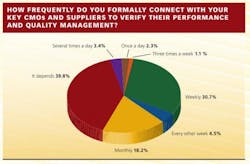
Communication, or the lack of it, has clearly become a factor in the overall CMO management picture. In the survey, 5% of respondents describe communicating with key contract partners at least once a day, 31% weekly, 18% monthly, but 40% answered with a vague “it depends.”
Relatively infrequent communication would appear to conflict with the stated goal of better managing knowledge and tech transfer, said Michael Long.
Pharmaceutical operating companies often fail to communicate adequately to their CMOs, as contract manufacturing companies reported in BioPlan’s 9th annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production [7]. Eighty-six percent of the 302 pharma operating company and CMO professionals who responded complained that biopharm clients didn’t build in enough time for projects, or communicate effectively, while 83% said they didn’t plan their tech transfer process or recognize variability in process development.
Another complaint: 67% of the CMO respondents to BioPlan’s survey said that their clients just “handed off a project” without planning for ongoing interactions. Some CMO respondents said that their pharma clients did not adequately use QA and QC expertise and expected CMOs to make regulatory decisions for them.
References
1. The U.S. Contract Manufacturing Outsourcing Market Revives as Pharmaceutical Companies Increasingly Outsource after the Recession, April, 2012.
http://www.frost.com/sublib/display-press-release.do?Src=RSS&id=257678521
2. Custom research from and communication with That’s Nice, LLC
3. Ninth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates,
p. 220-222
4. Long, M., Introduction to Quality By Design for Suppliers:
The First Steps
5. Russo, A., Outsourcing as a central success factor w.kpmg.com/CH/en/Library/KPMG-in-the-Media/Documents/pa-20120410-outsourcing-as-key-success-factor-for-the-future-of-the-pharmaceutical-industry-en.pdf
6. Rees, H., Supply Chain Management in the Drug Industry: Delivering Patient Value for Pharmaceuticals and Biologics.
http://www.amazon.com/Supply-Chain-Management-Drug-Industry/dp/0470555173
7. BioPlan op cit, p. 222