Real-Time Viable Particle Detection: Benefits, Challenges, and Regulatory Needs
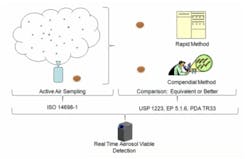
Rapid Microbiological Methods (RMM) have intrigued pharmaceutical manufacturers for several years. The benefits of RMM are well established; they enable better insight into the manufacturing process by providing microbial information much faster than the compendial methods that take multiple days to provide results. Most currently available RMM instruments are laboratory-based and significantly reduce the time to obtain microbial results from a collected sample. Several manufacturers have recently introduced real-time viable particle detectors similar to the BioTrak Real-Time Viable Particle Detector shown in Figure 1.
Real-time viable particle detectors use optical techniques to determine particle viability on a particle-by-particle basis. This capability provides for:
- trending of microbial content in the manufacturing environment;
- instantaneous notification of microbial contamination events;
- enabling segregation of potentially exposed product
- rapid initiation of root cause investigations
- providing input to Process Analytical Technology (PAT) based process control measures.
- potential for real-time product release
While real-time viable particle counters offer significant potential benefits, they also present some new challenges to industry and regulators. This article will address the unique challenges associated with evaluating, testing, and validating this new family of RMM instruments.
Viable Particle Detector
Real-Time Viable Particle Detector: Principles of Operation
Real-time viable particle detectors use the intrinsic fluorescence of microbial constituents associated with cell viability to analyze environmental particles entrained in the aerosol sample flow path. When excited by ultra violet laser light, the metabolites associated with cell viability fluoresce and emit light at a higher wavelength than the excitation wavelength. Principal microbial fluorophores associated with cell viability are tryptophan (excitation peak ~280 nm, emission peak ~340 nm), NADH (excitation peak ~340 nm, emission peak ~450 nm), and riboflavin (broad excitation ~350-450 nm, emission peak ~530 nm).
The most common excitation source for portable viability detectors is the 405 nm laser diode. The technique is non-specific. Organism specificity is not obtainable due to the complex composition of the viability metabolites and the resulting fluorescence signal. Laser induced fluorescence (LIF) has been used in military and homeland defense threat detection products since the late 1990’s. It has only recently been adapted for use in the pharmaceutical manufacturing environment.
Real-time viable particle detectors have several key operating parameters:
- Sample Flow Rate
- Aerosol Efficiency
- Sensitivity
- False Positive Rate
Sample flow rate is the amount of air that the instrument analyzes. Higher flow rates enable better characterization of manufacturing environments and reduce the time required to meet mandated sample volume requirements. Aerosol efficiency is the ratio of the number of particles present in the manufacturing environment compared to the particles that reach the instruments analysis engine. Sensitivity and False Positive Rate describe the ability of the real-time viable particle detectors to measure and differentiate viable particles from non-viable particles. Sensitivity is the ability to measure low numbers of viable particle counts. A false positive occurs when a non-viable particle is classified as a viable particle. False positives can occur due to the non-specific nature of the LIF technique. Additionally, non-viable particles such as pollens, skin flakes, and paper dust have fluorescence properties and create optical signals that must be addressed during instrument design. Typically, higher sensitivities result in higher levels of false positives. These key operating parameters should be considered and addressed when evaluating and validating real-time viable particle detectors.
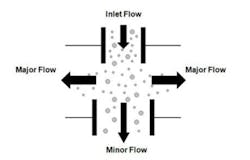
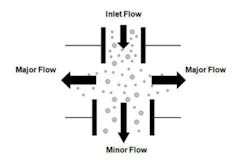
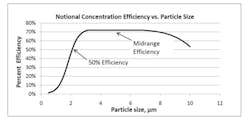
Due to the low intensity of the fluorescence signals, real-time viable particle detectors that have sample flows greater than approximately 5 liters/minute must reduce the flow to increase the fluorescence emission intensity. Aerosol concentration is the enabling technology incorporated into high flow rate detectors. Aerosol concentrators utilize particle inertia to concentrate the larger particles of interest into a lower velocity flow volume. The incoming sample flow is separated into a high (major) volume flow that is exhausted from the system and a low (minor) volume flow that is analyzed. Small low-inertia particles follow the high-volume flow path while larger high-inertia particles follow the low-volume analyzed flow path. Figure 2 illustrates the operating principles of a concentrator.The D50 is the particle size where 50% of the particles contained in the incoming flow are carried forward into the low volume analyzed flow. The efficiency is the percentage of incoming particles above the D50 carried forward into the low volume analyzed flow. The diversion of smaller particles into the exhaust flow is not a major concern since the majority of biological particles range in size from 2 to 10 microns. The efficiency is an important parameter since not all the particles in the incoming sample are analyzed, their numbers are reduced by the efficiency of the concentrator. Active air samplers are characterized in the same manner.
The detection efficiency of the viable particle detector is as equally important as its aerosol efficiency. All real-time viable particle detectors on the market today are based on intrinsic fluorescence. Intrinsic fluorescence is non-specific due to the variability of the quantity of fluorescent metabolites in microbiological organisms. Adding to the complexity of the viable particle detection is the fact that some non-viable particles also have fluorescence properties. The detection performance of viable particle detectors is usually a tradeoff between its sensitivity (i.e. its ability to detect low levels of viable particles) and the amount of false positives that it generates when exposed to naturally occurring environments comprised of both viable and non-viable particles.
Currently available viable particle detectors use different analysis techniques and incorporate different optical input parameters. Thus, different instruments will have different performance characteristics in terms of sensitivity and false positives. Sensitivity and false positive performance is extremely difficult to characterize in controlled laboratory conditions due to the variability of particles that are present in the manufacturing environment. Adding to the uncertainty are the known deficiencies of the compendial method: which agar should be used, what incubation temperature and time should used, and the recognition that Viable But Non Culturable (VBNC) organisms exist.
Regulatory Guidance
With the exception of real-time viable particle detectors, the majority of current RMM instruments are operated in the microbiology lab and speed up the process of analyzing microbial content of previously obtained environmental or product samples. The guidance given in USP 1223, EP5.1.6, and PDA TR33 was developed based on laboratory test methodologies. The key evaluation criteria given in TR 33, USP 1223 and EP 5.1.6 consist of:
- Accuracy
- Precision
- Specificity
- Detection Limit
- Quantification Limit
- Linearity
- Operational Range
- Robustness
- Repeatability*
- Ruggedness*
- How the aerosol is introduced during routine operation as well as laboratory based evaluation
- The aerosol efficiency of the real-time viable particle detector
- The aerosol efficiency of the active sampler used to obtain the comparison sample
- The detection efficiency of the real-time viable particle detector
- The false positive rate of the real-time viable particle detector
Current RMM guidance does not appropriately address the critical performance parameters of real-time viable particle detectors. As mentioned previously, current guidance focuses on comparing new laboratory based methods to the compendial culture based method. This guidance addresses the majority of post sample collection laboratory based RMM instruments. The established technique of serial dilution is appropriate for characterizing the key performance characteristics defined in the guidance. Real-time viable particle counters gain their utility from measuring aerosol viable particles. This introduces a significant source of uncertainty into the evaluation process. The uncertainty involves both the ability to generate well-controlled challenge aerosols in addition to having the aerosol efficiency of both the active air sampler and the real-time viable particle detector well characterized. Finally, how the challenge aerosol is introduced to the real-time viable particle detector is critical and can have a large influence on the reported results.
Establishing the Limit of Detection (LOD) is a challenging task for several reasons. It is extremely difficult to generated stable low concentrations of aerosolized viable particles. Additionally, the LOD of an aerosol instrument is determined by count and sampling statistics. The LOD is influenced by the sample volume, sampling time, detection efficiency, and the relative aerosol efficiency of both the reference aerosol collector and the real-time viable particle detector under test. It is difficult to apply and evaluate single particle detection to aerosol instrumentation. There are aerosol generation methods that can generate single particle challenges. Single particle generation equipment produce a series of single particles that can be directed into the inlet of the real-time viable particle detector and measured allowing single organism capability to be claimed. It should be noted that this test methodologies do not correspond to how the instrument will operate in the manufacturing environment. Evaluating LOD and single particle detection capability requires a discussion of sampling statistics, flow volumes, and particle concentrations in order to establish the correlation between the laboratory experiment and real-world performance.
Current RMM guidance does not adequately address evaluation and validation of real-time viable particle counters. The guidance criteria are generally applicable, but the test methodologies do not adequately address the unique characteristics and challenges associated with aerosol instrumentation.
The key performance criteria given in USP <1223>, EP 5.1.6 and PDA TR33 are applicable, but the test methodology and performance limits require refinement and clarification. The challenges associated with evaluating this new family of RMM instrumentation are directly related to the benefit they offer to the manufacturing process: the ability to measure for the presence of viable organisms on a real-time basis. The FDA aseptic processing guidelines states: “Manufacturers should be aware of a device’s air monitoring capabilities, and the air sampler should be evaluated for its suitability for use in an aseptic environment based on collection efficiency, clean ability, ability to be sterilized, and disruption of unidirectional airflow” (Guidance for Industry Sterile Drug Products Produced by Aseptic Processing-Current Good Manufacturing Practice FDA 2004). Similar awareness and understanding needs to be developed for real-time viable particle detectors. Characteristics such as sample flow rate, aerosol efficiency, sensitivity, and the false positive rate should be considered and evaluated. In order for the full benefit of this new technology to be realized, collaboration between vendors, industry, and regulatory authorities is required to define and implement better test methodologies to characterize and validate them.
Conclusion
The ability to monitor for the presence of viable particles on a real-time basis offers tremendous benefit to the pharmaceutical manufacturing process. The real-time aerosol-based viability measurement does not lend itself to current RMM guidance developed for post sample collection laboratory-based instruments. Methodologies that consider the unique nature of the measurement must be developed through active dialogue between instrument vendors, industry, and regulatory bodies. The benefit offered by this new family of RMM instruments is well worth the effort.
About the Author
Darrick Niccum is a Senior Global Product Manager-Biotechnology for TSI Incorporated. He has been involved in development of particle detection instrumentation, including fluorescence based viability detections for over 12 years. TSI Incorporated, 500 Cardigan Road, Shoreview MN 55126. Questions can be directed to [email protected].