Control Cross-Contamination Risk with HAPIs: Using a Risk-MaPP Master Matrix
Recently, an international regulatory agency challenged Elan Drug Technologies’ manufacturing services business, now part of Alkermes plc, to develop an approach for managing risk at its multiproduct facility in Athlone, Ireland. The plant processes many different active pharmaceutical ingredients (APIs), including high potency APIs (HAPIs).
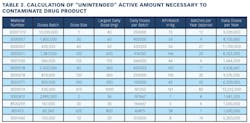
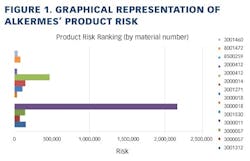
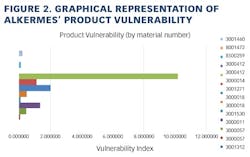
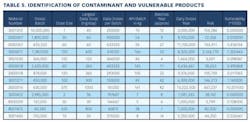
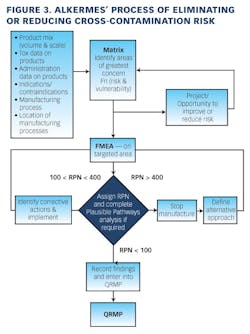
Working closely with PharmaConsult Ltd.’s Stephanie Wilkins, cochair of ISPE’s Risk-MaPP task team, the facility responded with a Master Matrix based on Risk-MaPP, a risk-based framework derived from principles outlined in ICH Q9. Risk-MaPP is designed to allow companies to identify and focus on critical risk areas to help prevent cross-contamination and ensure that controls applied are appropriate and commensurate to the risk. The Master Matrix, in turn, is a spreadsheet that offers a visually effective way to see where cross-contamination risks, both process-related and product-related, are highest, allowing appropriate actions to be taken. This tool allows Alkermes to assign a numeric value, not only to each potential source of cross-contamination risk, but also to products that are vulnerable to cross contamination.This article will briefly summarize how the Master Matrix was implemented and what results it has shown so far.Alkermes considers the “opportunity to contaminate” as a function of the frequency of the given product’s manufacture. In addition, the company characterizes source batches or dosage forms at risk of cross-contamination by evaluating the following:• toxicity • quantity of active used per batch• process train used in product manufacture • level of containment and the energies employed in processing• proximity to other products and the use of shared equipment • opportunity to contaminate • dosing regime of the product and in particular the number of daily doses contained in a batch• frequency of the ingredient’s or product’s manufacture• any other products manufactured on site that might be contra-indicated for users of the target drug.The goal is to identify and highlight points at which high risk and high vulnerability coexist. The elements of the matrix can be divided into five subsections or “elements.”1. Product DataThe first step in building the Master Matrix was to list products manufactured on site. Internally, to simplify communication, product names were used, while externally (and below) product numbers were employed to ensure confidentiality (See Table 1).- Everything is related to a finished batch size; where multiple components are combined in that final batch, multiplication of each individual component score by the number of components combined in a batch is undertaken. For example, coated beads are manufactured in the CF granulators in component batch sizes of 60-80 kg, but finished (encapsulation) batch sizes are bulked up by combining four of these in a blend. As a result, a 4x “component score” is entered in the matrix.
- A number of Alkermes processes are iterative. Again, taking the example of our CF coated beads, typically multiple layers are used. The first is when active material is coated directly onto a non-pareil core. Such a process is “semi-open” with “medium to high” energy input and is scored a 12. The next coating is of a polymer onto the active core—the potential for active “release” exists but is much reduced as all active has adhered to the bead rather than existing in a powder state and auger feed to an agitated bed. Further, as polymer coating covers more and more of the bead surface, the product design virtually eliminates potential for contaminant generation. The second coat is scored a 10 and all subsequent coats as 5 or 2. All scores from the individual iterations are then combined.
About the Authors
Mark O’Reilly joined Alkermes (formerly Elan Drug Technologies) in 1993. He is currently Senior Director of Engineering where his responsibilities include process train and facility design, industrial hygiene and validation. He received his BE from University College Dublin, his MEngSc from the University of New South Wales and his MSc from the University of Manchester.
Aisling Horan joined Alkermes (formerly Elan Drug Technologies) in 2003. She currently works in the validation department with a primary focus on process & cleaning validation and cross contamination. In addition to validation, her experience includes industrial and clinical microbiology. Aisling received her BSc (Hons) in microbiology from the National University of Ireland, Galway (NUIG).