A Business Case for Isolation Technology
Jacobs Engineering regularly counsels clients on sterile facility design and on the relative merits of differing kinds of aseptic areas. Expense is obviously critical, so Jacobs recently conducted a study to get a better grasp of how traditional cleanrooms compared with the more recent RABS (restricted access barrier systems) and isolation technologies in terms of ROI. (To view the poster presented at ISPE 2009, click here.)
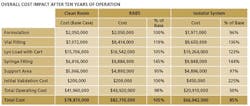
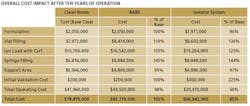
The key finding: Though capital costs for isolator-based facilities are higher upfront, they tend to be cheaper in the long run (see Table below). We corresponded with three of the study leads—Kevin Schreier, Manager of Process Engineering, Jorge Ferreira, Technology Manager, and Beth Holden, Architect.
PhM: How extensive and reliable is the data? How many different vendors did you consult with?
J.E.: All data was derived from our database of recent Jacobs projects, which includes equipment pricing from multiple vendors. The facility and operating costs have been estimated using the same methodology, assuming a basis of East Coast U.S. cost data, and are a relative comparison to illustrate the difference between each option.
PhM: And what kind of modeling did you do?
J.E.: The facility model we used in this analysis borrows from other modeling tools we have developed. We have generated standard design tools, including generic room layout templates for specific unit operations (Kit of Parts) and benchmark databases (Class of Plant), permitting us to compare project impacts when the level of technology is modified. Combining these and other tools, we generated a set of standard modules to define a comparison of the capital costs. To complete the analysis, we then added a comparison of the operating costs for each technology.
PhM: Of all the data from the study, what specifics jump out at you the most? Is there anything that’s making you rethink how you approach projects requiring aseptic processing?
J.E.: In the past, we have been uncertain of the actual benefits of RABS technology. Although it was perceived to be a less capital-intensive technology, we have seen that it costs more initially and, in the long term, to operate, than a traditional cleanroom.
PhM: The isolated filling line has an initial capital cost 24% above RABS and conventional clean rooms. What accounts for this difference, and are there ways that manufacturers can reduce initial capital costs with isolators?
J.E.: There is an increased level of complexity in isolators over clean rooms and additional perceived requirements for qualification and validation. We invite the isolator manufacturers to comment on equipment costs but suggest that since the technology is maturing and the development process leveling off, innovation will likely lead to a reduction in equipment costs.
PhM: You note that, over 10 years, isolation technology more than pays for itself. Is there a specific time (eg, 5-7 years out?) that you would indicate as a break-even point?
J.E.: In this analysis, the three technologies were compared against a common set of assumptions, in order to understand the relative long term operating costs. Using our approach, we can calculate a specific break-even point for a facility from a specific set of conditions. For this example, the break-even would occur at the end of 6th year, but it is expected to vary depending upon project specific parameters.
PhM: The reduced cost of environmental monitoring is one way that isolator lines are able to “make up ground” and save over time. Is this generally true for all projects, or can this number vary significantly?
J.E.: Environmental monitoring locations are typically a combination of anticipated “problem” areas and a square root function of the process area. Reducing the process area through the use of an isolator does not result in an equal reduction in the number of sampling locations.
PhM: Utility costs also favor isolators over RABS and cleanrooms. How much of this is dependent on energy costs, and did you factor in uncertainty in utility rates into your study?
J.E.: In this analysis, operating costs were kept constant over the 10 years. It can be surmised that if operating costs escalate over the lifecycle, the differential savings demonstrated by isolation technology would be increased.
PhM: Did you find anything to suggest that validation costs should be more, or less, of a factor in decisions to retrofit facilities or install new isolated filling lines?
J.E.: Experience shows that validation costs for isolators could be much higher than cleanrooms, but from a project perspective the validation costs associated with the isolator or cleanroom installation are only about 1% of the total project costs, and, therefore not likely to impact the decision to use isolation technology. However, the perceived complexity of isolator validation may be a deterrent to some companies that are accustomed to conventional cleanrooms.
PhM: Has this study changed the advice that you might give clients, perhaps giving more credence to the argument to pursue isolators over RABS or clean rooms?
J.E.: Unfortunately no, each project typically has a unique set of circumstances that guides the direction and technology best suited for the application. There will still be a need for RABS and clean rooms in the future and one never knows . . . maybe soon we’ll have mini-disposable environments to fill novel drugs or isolated pandemic responses, to add to the evaluation.