Tablets are the most common drug dosage form today, and thus granulation, which allows primary powder particles to adhere and form granules, is one of the most important unit operations in drug manufacturing. Understanding granulation grows more complex each year. This article reviews the most current methods and mechanisms of pharmaceutical granulation, including factors that can lead to improved control.
Particle-bonding Mechanisms
a) Adhesion and cohesion forces in immobile films. If sufficient liquid is present in a powder to form a thin, immobile layer, there will be an increase in contact area between particles. The bond strength between particles will increase, as the Van der Waals forces of attraction are proportional to the particle diameter and inversely proportional to the square of the distance of separation [1].
b) Interfacial forces in mobile liquid films. During wet granulation, liquid is added to the powder mix and distributed as films around and between the particles. There are three states of water distribution between particles. At low moisture levels, the pendular state, particles are held together by surface tension forces of the liquid/air interface and the hydrostatic suction pressure in the liquid bridge.
When all the air has been displaced from between the particles, the capillary state is reached, and the particles are held by capillary suction at the liquid/air interface. The funicular state represents an intermediate stage between the pendular and capillary states. Moist granule tensile strength increases about three times between the pendular and the capillary state. These wet bridges are, however, a prerequisite for the formation of solid bridges formed by adhesives present in the liquid, or by materials that dissolve in the granulating liquid.
Solid bridges can be formed in two ways:
Hardening binders. When an adhesive is included in the granulating solvent it forms liquid bridges, and the adhesive will harden or crystallize on drying to form solid bridges to bind the particles.
Crystallization of dissolved substances. The solvent used to mass the powder during wet granulation may partially dissolve one of the powdered ingredients. When the granules are dried, crystallization of this material will take place and the dissolved substance then acts as a hardening binder.
c) Attractive forces between solid particles. In the absence of liquids and solid bridges formed by binding agents, there are two types of attractive force that can operate between particles in pharmaceutical systems, electrostatic forces and Van der Waals forces. Van der Waals forces are about four orders of magnitude greater than electrostatic and add to the strength of granules produced by dry granulation.
Mechanisms of Granule Formation
a) Nucleation. Granulation starts with particle-particle contact and adhesion due to liquid bridges. A number of particles will join to form the pendular state. Further agitation densifies the pendular bodies to form the capillary state, and these bodies act as nuclei for further granule growth [2].
b) Transition. Nuclei can grow in two possible ways: either single particles can be added to the nuclei by pendular bridges, or two or more nuclei may combine. The combined nuclei will be reshaped by the agitation of the bed. This stage is characterized by the presence of a large number of small granules with a fairly wide size distribution.
c) Ball Growth. If agitation is continued, granule coalescence will continue and produce an unusable, over-massed system, although this is dependent upon the amount of liquid added and the properties of the material being granulated [1].
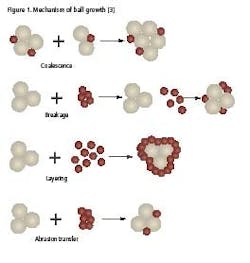
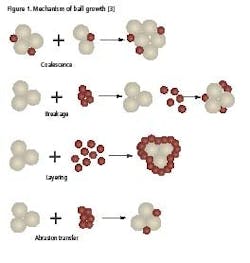
There are four possible mechanisms of ball growth, which are illustrated in Figure 1 [3]:
- Coalescence. Two or more granules join to form a larger granule.
- Breakage. Granules break into fragments which adhere to other granules, forming a layer of material over the surviving granule.
- Layering. When a second batch of powder mix is added to a bed of granules, the powder will adhere to the granules, forming a layer over the surface and increasing the granule size.
- Abrasion Transfer. Agitation of the granule bed leads to the attrition of material from granules. This abraded material adheres to other granules.
Granulation Methods [4]
Dry Granulation. This requires two pieces of equipment, a machine for compressing the dry powders into compacts or flakes, and a mill for breaking up these intermediate products into granules. The dry method may be used for drugs that do not compress well after wet granulation, or those which are sensitive to moisture.
Wet Granulation. In this method, the wet mass is forced through a sieve to produce wet granules which are then dried. A subsequent screening stage breaks agglomerates of granules. Organic solvents are used when water-sensitive drugs are processed, as an alternative to dry granulation, or when a rapid drying time is required. Because direct compressing is not the best technology for many active substances, wet granulation is still a preferred method. Even if the active substance is sensitive to hydrolysis, modern equipment (e.g., a fluidized bed) eliminates all problems in wet granulation [2].
Factors Affecting Granulation Methods
Liquid Requirement. High-shear mixers may exhibit a narrow margin between the liquid required to obtain granule growth and the amount that results in an over-wetted mass. Because of the intensive wet massing and densification of the granules, less liquid is normally required with high- than with low-shear mixers [5]. In addition, impeller rotation speed influences the liquid requirements, as does evaporation of the solvent, usually water, in the binder solution. Especially with high-shear mixers, intense agitation results in a temperature rise and loss of solvent by evaporation.
Effects of Raw Material Properties. The following properties influence granule formation and growth:
- Contact angle of the binder liquid to the solids
- Solubility of the particles in the binder liquid
- Mean particle size and size distribution of the solids
- Particle shape and surface morphology
- Packing properties of the solids
Raw materials must have good wetting properties if there is to be uniform liquid distribution and, hence, controlled granule growth. The smaller the particle size of the raw material, the more binder liquid required.
Binder Properties
Binder Concentration. The binder forms an internal matrix; consequently, the granule strength and tablet strength increase as binder concentration increases. Mechanical Properties of Binder. The mechanical properties of the binder determine binder strength and deformation behavior of the binder matrix.
Properties of Drug and Other Excipients in the Formulation
Wet granulation depends upon wetting of powder by the binder solution, surface tension of lenticular bridge films formed and solution viscosity. Binder Distribution. The distribution influences the binder’s ability to produce strong and non-friable granules. The processing method used to distribute the binder influences binder efficiency.
Endpoint Determination
Endpoint can be defined as a target particle size mean or distribution.
Traditional Methods
a) Power Consumption. Power consumption of the mixer motor for end-point determination and scale-up is widely used because the measurement is economical, does not require extensive mixer modifications and is well correlated with granule growth [14]. Intragranular porosity also shows some correlation with power consumption. Normalized work of granulation (power profile integrated over time) can accurately determine endpoints and is correlated well with properties of granulates.
b) Impeller Torque. Direct torque measurement requires installation of strain gauges on the impeller shaft or on the coupling between the motor and impeller shaft. Since the shaft is rotating, a device called a slip ring is used to transmit the signal to the stationary data acquisition system.
c) Torque Rheometer. A torque rheometer provides an off-line measurement of torque required to rotate the blades of the device and can be used to assess rheological properties of the granulation. It has been extensively used for endpoint determination. The torque values obtained have been termed a “measure of wet mass consistency” [15].
d) Reaction Torque. As the impeller shaft rotates, the motor tries to rotate in the opposite direction, but does not because it is bolted in place. The tensions in the stationary motor base can be measured by a reaction torque transducer.
e) Other Possibilities. When agglomeration is progressing very rapidly, neither power consumption nor torque on the impeller may be sensitive enough to adequately reflect material changes. Some investigators feel that other measurements, such as torque or force on the impeller blades, may be better suited to monitor such events. There are other ideas floating around—for example, use of neural networks to describe and predict the behavior of the wet granulation [16] or control of the endpoint by a rapid image processing system [17]. A technique for measuring tensile strength of granules, in addition to power consumption measurement, to facilitate optimal endpoint determination, has been described by Betz, Bürgin and Leuenberger. Powder flow patterns in wet granulation can be studied using positron emission particle tracking [18].
Emerging Technologies
a) Acoustic. Applicability of piezo-electric acoustic emission sensors to endpoint determination has been studied since the beginning of this century [19]. The technique is very promising, especially since it is non-invasive, sensitive and relatively inexpensive. Granulation process signatures obtained with an acoustic transducer can be used to monitor changes in particle size, flow and compression properties.
b) Near-Infrared (NIR). Use of a refractive NIR moisture sensor for endpoint determination of wet granulation has been described by several authors [20]. There are technological challenges associated with this approach, as the sensor can only measure the amount of water at the powder surface.
c) FBRM. Focused beam reflectance measurement (FBRM) is a particle-size determination technique based on a laser beam focusing in the vicinity of a sapphire window of a probe [21]. The beam follows a circular path at speeds of up to 6 m/s. When it intersects with the edge of a particle passing by the window surface, an optical collector records a backscatter signal. The time interval of the signal multiplied by the beam speed represents a chord length between two points on the edge of a particle. The chord length distribution (CLD) can be recalculated to represent either a number or volume-weighted particle size distribution. In many cases, CLD measurements are adequate to monitor dynamic changes in process parameters related to particle size and shape, concentration and rheology of fluid suspensions.
Processing-Induced Transformations in Wet Granulation
The physicochemical properties of pharmaceuticals, including solubility and dissolution rate, can be influenced by the degree of crystallinity, solvation state and crystal form [22]. Problems related with wet granulation include:
Hydrate Formation. For example, theophylline has a low aqueous solubility (8 mg/mL at 25°C) and can exist as an anhydrate or as a monohydrate [23]. During pharmaceutical manufacturing, the stable anhydrous polymorph undergoes multiple transformations (stable anhydrate → hydrate → metastable anhydrate). Tablets prepared using the metastable form dissolve at a slower rate than those containing the stable polymorph. This difference is attributed to rapid metastable anhydrate → theophylline monohydrate conversion during dissolution. Thus, processing-induced phase transformations significantly influence the dissolution behavior of theophylline tablets. For online monitoring of the transformation from one form to another, Raman spectroscopy is most widely used.
Polymorphic Transformation. Glycine, for example, exists in three polymorphs: α, β, and γ . Among them, γ is the most stable form and α is the metastable form. The stable glycine polymorph γ converts to metastable form α when wet granulated with microcrystalline cellulose. NIR is used to monitor the blending of the starting materials. Raman spectroscopy, NIR, and x-ray powder diffraction have been used in the characterization of polymorphic changes during the process.
References
- Henning, G, Torben, S. Granulation, In: Encyclopedia of Pharmaceutical Technology. Marcel Dekker. New York. 121-144 (1997).
- Augsburger, L.L., Vuppala, M.K., In : Parikh, D.M. Handbook of Pharmaceutical Granulation Technology. Marcel Dekker. New York, 81 (1997).
- Summers, M.P. Granulation, In: Pharmaceutics, the Science of Dosage Form Design Churchill Livingstone. New York. 616-628 (1988).
- Das, S, Jarowski, C. Effect of granulating method on particle size distribution of granules and disintegrated tablets. Drug Dev. Ind. Pharm.
- 479 (1979). 5. Yliruusi, J, Tihtonen, R. High-speed granulation. Part I: Effect of some process parameters of granulation on the properties of unsieved and wet-sieved granules. Acta Pharm Fen. 98: 39 (1989).
- Paschos, S, Cognart, J, Jeannin, C, et al. Granulation with a highspeed mixer-granulator-dryer: Optimization of the process. Acta Pharm. Technol. 34: 80 (1988).
- Cliff, MJ. Granulation end-point and automated process control of mixergranulators: Part 1. Pharm Tech. 4: 112-132 (1990).
- www.ptemagazine.com. Accessed February 14, 2007.
- Worts, O. Wet granulation – fluidized bed and high shear techniques compared. Pharm. Tech. Europe. 10(11): 27-30 (1998).
- Satoru, W., Yasushi, I., Kenji, H., et al. Microgranulation of fine powders by a novel rotating fluidized bed granulator. Powder Technology. 131: 250- 255 (2003).
- www.rocw.raifoundation.org/biotechnology. Accessed February 5, 2007.
- www.pharmainfo.net. Accessed February 5, 2007.
- Thoma, K., Ziegler, I. Investigations on the influence of the type of extruder for pelletization by extrusion–spheronization I. Extrusion behavior of formulations. Drug Dev. Ind. Pharm. 24(5): 401-411 (1998); II. Sphere characteristics. Drug Dev. Ind. Pharm. 24(5): 413-422 (1998).
- Holm, P., Schaefer, T., Larsen, C. End-point detection in a wet granulation process. Pharm Dev Technol. 6(2): 181-192 (2001).
- Faure, A., Grimsey, I.M., Rowe, R.C., et al. Applicability of a scale-up methodology for wet granulation processes in Collette Gral high shear mixergranulators. Eur J Pharm Sci. 8(2): 85-93 (1999).
- Watano, S., Sato, Y., Miyanami, K. Application of a neural network to granulation scale-up. Powder Technol. 90(2): 153-159 (1997).
- Watano, S. Direct control of wet granulation processes by image processing system. Powder Technol. 117(1-2): 163-172 (2001).
- Laurent, BFC. Structure of powder flow in a planetary mixer during wet-mass granulation. Chem Eng Sci. 60(14): 3805-3816 (2005).
- Belchamber, R. Acoustics—a process analytical tool. Spec Eur. 15(6): 26-27 (2003).\
- Otsuka, M., Mouri, Y., Matsuda, Y. Chemometric evaluation of pharmaceutical properties of antipyrine granules by near-infrared spectroscopy. AAPS Pharm Sci Tech. 4(3): 47 (2003).
- Ganguly, S., Gao, J.Z. Application of on-line focused beam reflectance measurement technology in high shear wet granulation. Contributed paper, AAPS 2005 General Meeting.
- Morris, K.R., Griesser, U.J., Eckhardt, C.J., and Stowell, J.G. Theoretical approaches to physical transformations of active pharmaceutical ingredients during manufacturing processes. Adv. Drug Deliv. Rev. 48: 91-114 (2001).
- Phadnis, N.V., Suryanarayanan, R. Polymorphism in anhydrous theophylline implications on the dissolution rate of theophylline tablets. J Pharm Sci. 86: 1256-1263 (1997).
- www.atacamalabs.com. Accessed February 11, 2008.
- www.powderpro.se/tech.html. Accessed February 11, 2008.
- www.keram.se/eng/pdf/freeze_granulation. Accessed February 11, 2008.
- www.dow.com/dowexcipients/applications/foam.htm. Accessed February 11, 2008.
- Walker, G. Bell, S. Andrews, G. Co-melt fluidised granulation (CMFG): improvements in drug bioavailabiliy characterised by DSC and raman spectroscopy. Bioengineering In Ireland Conference, January 27-28, 2006.
- www.pharmpedia.com /Tablet:Manufacturing_methods/Granulation. Accessed February 19, 2008.
- www.in-pharmatechnologist.com/marketreport. Accessed February 19, 2008.
About the Authors
Dr. Rakesh Patel is assistant professor in the department of Pharmaceutics & Pharmaceutical Technology at Shree S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Gujarat, India. He can be contacted at [email protected]. Mr. A. M. Suthar is pursuing his M. Pharm. in Industrial Pharmacy at the S. K. Patel College of Pharmaceutical Education and Research.