Many pharmaceutical manufacturers are adopting Lean manufacturing practices and investing millions of dollars on supply chain software and other initiatives to streamline and expedite the flow of goods. They’re attempting to cut costly production delays, inefficient processes and slow movement of goods to bring life-saving products to market.
Saving 30 minutes a shift, multiplied by the number of working days and the number of plants, can add up to significant annual savings. Pharmaceutical manufacturers who regularly test for microbiological contamination in raw materials, inprocess or finished goods can literally cut days out of their cycle times by adopting rapid microbial methods (RMM).
At the same time, RMM can ensure equal or better raw materials and product quality standards, and is becoming a more widely accepted method of regulatory compliance. The micro hold area is becoming the last great frontier for reducing process time and waste. Most manufacturers of pharmaceutical products regularly sample and test their raw materials or finished goods for microbiological contamination.
Most of them are using an outdated and inefficient process dating back to the 1800s that takes three, five, seven or more days to yield results. During this waiting period, the full lots of raw materials or finished goods being tested are often quarantined in micro-hold. Reducing the time that any product spends in microhold increases supply chain efficiency and customer responsiveness, freeing valuable resources and liquidity that could otherwise contribute to profitability.
When inventory isn’t getting off the floor and out the door fast enough, revenue is lost – and ultimately patient safety is compromised. Consider how much a company might spend to relocate processes on the manufacturing floor just to save 10 minutes of transport time.
What would it be worth to save three or more days per batch? That’s when the benefits of rapid methods become clear. While the majority of pharmaceutical manufacturers have evaluated RMM, only three percent of production facilities globally have installed rapid micro systems as of 2004. By this year (2008), adoption is expected to grow to 10 percent.
Using RMM for testing raw materials, in-process and finished goods, pharmaceutical manufacturers can better meet or improve upon product quality standards, help satisfy regulatory compliance policies and reap measurable financial gains.
Available Rapid Microbial Methods
Several types of RMM technologies are available to pharmaceutical manufacturers. These include methods which enhance the growth of microorganisms by incubating samples in an enrichment broth so that growth can be detected sooner. The time required to detect depends on the technology used. Impedance is one method that serves as a presence/absence test.
As microorganisms grow in their nutrient broth, they metabolize and therefore change the chemical nature of the broth. This results in a change in electrical current that can be detected by the technology. Most impedance methods can produce a fairly rapid result, but only if the sample is positive and the manufacturer wants to detect bacteria or yeast, not mold.
Another growth-based rapid microbial method is flow cytometry. This technology utilizes a combination of cell labeling and laser excitation to detect living (contaminated) organisms. Flow cytometry is best suited for clear liquid products in water, narrowing the product range of candidates which can be tested. ATP bioluminescence is yet another RMM approach. When the enzymes luciferase and luciferin come in contact with the molecule ATP, which is present in all organisms, the result will be an emission of light directly proportional to the amount of ATP present in a sample.
A luminometer measures the light. An enhanced rapid detection systems use highly sensitive adenylate kinase (AK) bioluminescence assays, which can cut waiting time by half or more. These next-generation RMM systems featuring newer enzyme and molecular-based technologies and significantly compress testing times to 18-24 hours, even for mold, vs. three to seven days with traditional methods.
With greater sensitivity and reproducibility, advanced RMM technology offers a distinct advantage. By reducing the time required to test and release goods, pharmaceutical manufacturing companies can drive new efficiencies throughout the supply chain and realize significant cost savings. This FDA-accepted technology also supports efforts to achieve certification with ISO 9001 standards, cGMP compliance and other manufacturing best practices.
Benefits of RMM vs. Agar Plates
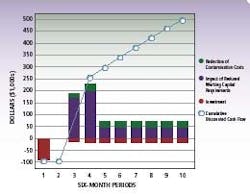
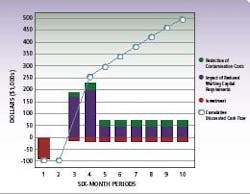
Irrespective of type, RMM technology has a distinct advantage compared to traditional detection methods using agar plates. With RMM, companies can significantly reduce manufacturing cycle times and increase throughput. Users no longer have to wait for results from incubations of three to seven days in the micro-hold area. The diagram (left) summarizes potential savings from RMM compared with traditional methods.
ROI,cost and savings data for a typical pharma RMM installation
By compressing the time required for micro-testing at various stages of production, pharmaceutical manufacturers can significantly streamline cycle times. The cumulative effect of these cycle time savings is substantial – up to eight days saved in the illustration based on an average five day micro-hold time, and more if testing is performed on in-process work.
Containing Contamination Saves Costs
Put simply, the results of microbial testing with RMM technology are now available to pharmaceutical manufacturers in just hours – not days. This represents a new “best practice” for these manufacturing companies whose livelihood depends on cycle time speed and supply chain efficiency. For example, the sooner a company knows that a batch is free of contaminants, the sooner it can release its held stock or batches. This enables manufacturers to bring products to market faster, which is a critical competitive benefit.
Since a controlled manufacturing environment will have uncontaminated products 95-99 percent of the time, a presence/absence screen can be an efficient and fast testing method. Yet if there is a contamination event, the sooner it is known the sooner it can be contained. Pharmaceutical manufacturers using RMM can initiate faster corrective action, reduce production recovery time, and provide for more rapid release of replacement product.
In the end, quicker response time minimizes the overall negative economic impact of discarded or reprocessed goods – saving companies not only money, but reputation.
RM – Fast Results, Quick Time to ROI
Implementing advanced RMM represents an average investment of less than $100k, which is typically recouped within 6-9 months of use. Further, the effects of reduced investment in quarantined inventory and more effective management of contamination events provides a cumulative discounted cash flow over time with a net present value of approximately $500,000 to $750,000 in five years.
This does not include a calculation for the additional benefits of reduced safety stock requirements (as a result of reduced manufacturing lead times) or further intangibles such as improved customer relations due to increased responsiveness.
Following are some examples of operational areas where savings can be quantified:
- Reduces working capital requirements – RMM can help lower the cost of capital by decreasing your investment in manufacturing, since it takes fewer days to produce the same amount of product. The shortened cycle time also reduces safety stock requirements as well as the amount of product held in quarantined inventory at a given time.
- Decreases stock carrying costs – What’s your interest rate for finished goods capital? RMM helps reduce the amount of capital tied up in finished goods inventory, and therefore shaves costs for carrying excess stock.
- Saves warehouse space – How much physical space is required in your warehouse or distribution center to store one day’s production of finished goods? To store inventory and safety stock? Calculate the cost per cubic foot of a single production day’s worth of warehouse space, then multiply that number by how many fewer days your product will spend in storage. Add this space-savings number to your product savings.
- Streamlines the supply chain – RMM drives finished goods to market faster, leading to quicker sales. This helps accelerate your revenue cycle.
- Improves recovery from contamination events – Rapid detection not only accelerates recovery time, but helps reduce the amount of contaminated product that must be scrapped or, worse yet, recalled from patients.
Faced with ever-increasing pressures to drive costs out of the supply chain while meeting customer demands, RMM offers pharmaceutical manufacturers another tool that can help them to speed products to market while ensuring that quality and safety standards are met.
About the Author
Nick Nichols is Vice President and General Manager of the Rapid Detection division of Celsis International plc. Celsis is a leading provider of innovative life science products and laboratory services to the global pharmaceutical, biopharmaceutical, and consumer products industries through its three business areas: Rapid Detection systems for industry, Analytical Services’ accredited testing labs, and drug discovery products and services from In Vitro Technologies. Headquartered in Chicago, the company is listed on the London Stock Exchange (CEL.L).